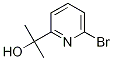
2-(6-broMopyridin-2-yl)propan-2-ol synthesis
- Product Name:2-(6-broMopyridin-2-yl)propan-2-ol
- CAS Number:638218-78-7
- Molecular formula:C8H10BrNO
- Molecular Weight:216.08
626-05-1

67-64-1

638218-78-7
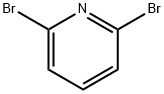
GENERAL STEPS: In a dry 250 mL round-bottomed flask equipped with a stir bar, septum and temperature probe, 1.6 M hexane solution of n-butyllithium (31.2 mL, 50 mmol) was added. The reaction system was cooled to -76 °C in a dry ice-acetone bath. Subsequently, THF (30 mL) was added to the reaction vial and a solution of 2,6-dibromopyridine (11.5 g, 50 mmol) in THF (60 mL) was added slowly dropwise by syringe, controlling the reaction temperature below -60 °C. Stirring of the dark yellow-brown solution was continued in a dry ice bath for 30 min, and then acetone (6 mL, 80 mmol) was added. The reaction mixture was stirred in a dry ice bath for 15 minutes and then slowly warmed to room temperature. After 1 hour of reaction, the reaction was quenched by careful addition of 5% aqueous ammonium chloride solution (50 mL). The reaction mixture was extracted with dichloromethane, the organic phases were combined and the solvent evaporated to give 2-(6-bromopyridin-2-yl)propan-2-ol (10.6 g, 98% yield) as an orange oil. The mass spectrum (MS) showed m/z: 216 and 218 (M+H)+.
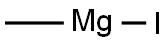
917-64-6
151 suppliers
$45.00/10ml
26218-75-7
236 suppliers
$9.00/1g

638218-78-7
101 suppliers
$37.00/100mg
Yield: 100%
Reaction Conditions:
in diethyl ether at 20; for 0.5 h;Inert atmosphere;
Steps:
1.1 Step 1: Preparation of 2- (6-bromopyridin-2-yl) propan-2-ol (Int-1-b)
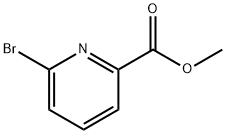
Dissolve methyl 6-bromopicolinate (Int-1-a) (5 g, 23.1 mmol) in diethyl ether (100 mL),Methyl magnesium iodide (17 mL, 50.8 mmol) was added under a nitrogen atmosphere, and the mixture was stirred at room temperature for 0.5 hours.Water and 2N hydrochloric acid were added to the reaction solution, and extracted three times with ethyl acetate.The organic layers were combined and washed successively with a saturated aqueous sodium hydrogen carbonate solution and a saturated saline solution.It was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain the title compound (5 g, yield: 100%) in this step.
References:
Sichuan Kelun Botai Bio-pharmaceutical Co., Ltd.;Liu Jinming;He Ting;Cai Jiaqiang;Chen Qiangqiang;Kang Xiwei;Wang Lichun;Wang Jingyi CN110467615, 2019, A Location in patent:Paragraph 0176-0180

626-05-1
506 suppliers
$9.00/10g

67-64-1
0 suppliers
$17.30/10ml

638218-78-7
101 suppliers
$37.00/100mg
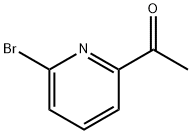
49669-13-8
227 suppliers
$5.00/250mg

75-16-1
275 suppliers
$12.00/10ml

638218-78-7
101 suppliers
$37.00/100mg

26218-75-7
236 suppliers
$9.00/1g

75-16-1
275 suppliers
$12.00/10ml

638218-78-7
101 suppliers
$37.00/100mg

49669-13-8
227 suppliers
$5.00/250mg
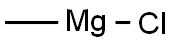
676-58-4
292 suppliers
$15.00/25ml

638218-78-7
101 suppliers
$37.00/100mg