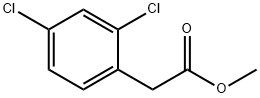
Methyl 2,4-dichlorophenylacetate synthesis
- Product Name:Methyl 2,4-dichlorophenylacetate
- CAS Number:55954-23-9
- Molecular formula:C9H8Cl2O2
- Molecular Weight:219.06

67-56-1
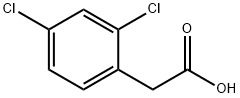
19719-28-9

55954-23-9
General procedure for the synthesis of methyl 2,4-dichlorophenylacetate from methanol and 2,4-dichlorophenylacetic acid: to a solution of 2,4-dichlorophenylacetic acid (3.5 g, 17.1 mmol) in methanol (100 mL) was slowly added concentrated sulfuric acid (20 drops). The reaction mixture was heated to reflux for 12 hours. Upon completion of the reaction, the solvent was removed by rotary evaporator under reduced pressure. The residue was diluted with cold water (50 mL) and extracted with ethyl acetate (3 x 50 mL). The organic layers were combined and washed sequentially with saturated sodium bicarbonate solution (50 mL) and saturated saline (50 mL), then dried with anhydrous sodium sulfate and filtered. The solvent was again removed by rotary evaporator under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography (eluent ratio 4:1 hexane/ethyl acetate) to afford methyl 2,4-dichlorophenylacetate (3.56 g, 95% yield) as a clear oil. The structure of the product was confirmed by 1H NMR (DMSO-d6): δ 7.40 (s, 1H), 7.22 (d, J=1.5 Hz, 2H), 3.74 (s, 2H), 3.71 (s, 3H).

19719-28-9
399 suppliers
$7.00/10g

74-88-4
357 suppliers
$15.00/10g

55954-23-9
123 suppliers
$9.00/5g
Yield: 100%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 21 h;
Steps:
5
Reference Example-5
After 2-(2,4-Dichlorophenyl) acetic acid (9.86 g, 48.1 mmol) was dissolved in DMF (50 mL), potassium carbonate (6.65 g, 48.1 mmol) was added thereto, and methyl iodide (21.6 g, 144 mmol) was added thereto, followed by stirring at room temperature for 21 hours.
After the reaction was completed, 2N hydrochloric acid (100 mL) was added to the reaction solution, and the resultant product was extracted with ethyl acetate (50 mL*1, 30 mL*2).
The organic layer was washed with a saturated saline solution (10 mL), dried over anhydrous magnesium sulfate, and concentrated under reduced pressure, whereby a brown oily crude product (15.1 g) was obtained.
This was purified by silica gel column chromatography (hexane:ethyl acetate=5:1), whereby methyl 2-(2,4-dichlorophenyl) acetate (10.7 g, yield: quantitative) was obtained as a colorless oily material. 1H-NMR (400 MHz, CDCl3): δ3.72 (s, 3H), 3.75 (s, 2H), 7.22 (d, J=1.2 Hz, 2H), 7.41 (t, J=1.2 Hz, 1H).
References:
KAKEN PHARMACEUTICAL CO., LTD.;SAGAMI CHEMICAL RESEARCH INSTITUTE;KOBAYASHI, Osamu;NIIKURA, Naoko;INOUE, Tomoko;MIZUTA, Satoshi;TAKATSUNA, Reiko;HIRAI, Kenji;SHIROUZU, Kentaro;OBATA, Miyoo US2016/24110, 2016, A1 Location in patent:Paragraph 0430; 0431

67-56-1
790 suppliers
$7.29/5ml-f

19719-28-9
399 suppliers
$7.00/10g

55954-23-9
123 suppliers
$9.00/5g