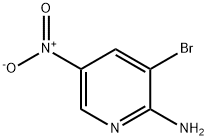
2-Amino-3-bromo-5-nitropyridine synthesis
- Product Name:2-Amino-3-bromo-5-nitropyridine
- CAS Number:15862-31-4
- Molecular formula:C5H4BrN3O2
- Molecular Weight:218.01
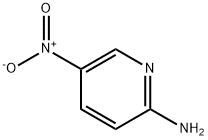
4214-76-0

15862-31-4
Step a: Bromine (38 g, 0.24 mol) was slowly added dropwise to a solution of 2-amino-5-nitropyridine (30 g, 0.22 mol) in acetic acid (200 mL) at 10 °C. After the dropwise addition was completed, the reaction mixture was warmed to 20 °C and stirring was continued for 30 min. Upon completion of the reaction, the solid product was collected by filtration and dissolved in ethyl acetate (200 mL). The pH of the mixture was adjusted to 8-9 with saturated aqueous sodium bicarbonate. the organic layer was separated and the aqueous layer was extracted with ethyl acetate (100 mL x 3). The organic layers were combined, washed sequentially with water and brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give 2-amino-3-bromo-5-nitropyridine (14.8 g, 32% yield). The product was characterized by 1H-NMR (CDCl3,400MHz): δ 8.94 (d, J=2.4Hz, 1H), 8.50 (d, J=2.4Hz, 1H), 5.67 (brs, 2H).

4214-76-0
522 suppliers
$6.00/10g

15862-31-4
246 suppliers
$5.00/1g
Yield:15862-31-4 32%
Reaction Conditions:
Stage #1: 5-nitro-pyridin-2-ylaminewith bromine;acetic acid at 20;
Stage #2: with sodium hydrogencarbonate in water;ethyl acetate; pH=8 - 9;Saturated solution;
Steps:
5.a
Step a: 3-Bromo-5-nitropyridin-2-amine To a solution of 5-nitro-pyridin-2-ylamine (30 g, 0.22 mol) in acetic acid (200 mL) at 10° C. was added Br2 (38 g, 0.24 mol) dropwise. After addition, the mixture was stirred at 20° C. for 30 min. The solid was filtered and then dissolved in ethyl acetate (200 mL). The mixture was basified to pH 8-9 with saturated aqueous NaHCO3. The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (100 mL*3). The combined organic layers were washed with water, brine, dried over Na2SO4 and concentrated under vacuum to afford 3-bromo-5-nitropyridin-2-amine (14.8 g, 32%). 1H-NMR (CDCl3, 400 MHz) δ 8.94 (d, J=2.4 Hz, 1H), 8.50 (d, J=2.4 Hz, 1H), 5.67 (brs, 2H).
References:
US2009/253736,2009,A1 Location in patent:Page/Page column 25
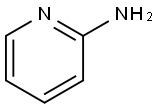
504-29-0
561 suppliers
$5.00/5G

15862-31-4
246 suppliers
$5.00/1g