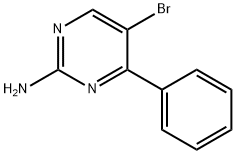
2-AMINO-5-BROMO-4-PHENYLPYRIMIDINE synthesis
- Product Name:2-AMINO-5-BROMO-4-PHENYLPYRIMIDINE
- CAS Number:85658-55-5
- Molecular formula:C10H8BrN3
- Molecular Weight:250.09
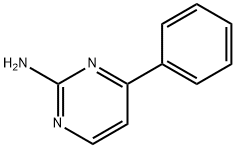
2305-87-5

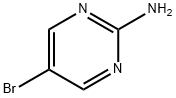
85658-55-5
To a 100 mL round bottom flask was added 4-phenylpyrimidin-2-amine (0.4 g, 0.0023 mol) and a solvent mixture of acetonitrile and chloroform (1:1, 20 mL). Subsequently, N-bromosuccinimide (0.499 g, 0.0028 mol) was added to the same flask. The reaction mixture was stirred at room temperature for 20 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure to give the residue. The residue was extracted by partitioning between ethyl acetate and water. The organic layer was separated, washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure to give the crude product. The crude product was purified by column chromatography using 60-120 mesh silica gel as stationary phase and 40% hexane solution of ethyl acetate as eluent to give 2-amino-5-bromo-4-phenylpyrimidine [0.3 g, 51% yield]. The product was confirmed by NMR hydrogen spectroscopy (300 MHz, DMSO-d6): δ 8.43 (s, 1H), 7.65-7.61 (m, 2H), 7.49-7.42 (m, 3H), 6.97 (brs, 2H); Liquid Chromatography-Mass Spectrometry (LC-MS) analysis showed that [M+2H]+ m/z 251.9.
7752-82-1
433 suppliers
$13.00/25g

98-80-6
746 suppliers
$5.00/5g

85658-55-5
44 suppliers
$45.00/25mg
Yield:85658-55-5 67%
Reaction Conditions:
with dipotassium peroxodisulfate in water;acetone at 160; for 0.75 h;Sealed tube;
References:
Thatikonda, Thanusha;Singh, Umed;Ambala, Srinivas;Vishwakarma, Ram A.;Singh, Parvinder Pal [Organic and Biomolecular Chemistry,2016,vol. 14,# 18,p. 4312 - 4320]

2305-87-5
96 suppliers
$14.14/250mgs:

85658-55-5
44 suppliers
$45.00/25mg

7752-82-1
433 suppliers
$13.00/25g
24388-23-6
207 suppliers
$6.00/5g

85658-55-5
44 suppliers
$45.00/25mg
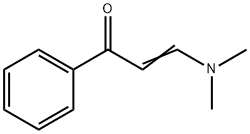
1201-93-0
96 suppliers
$17.00/250mg

85658-55-5
44 suppliers
$45.00/25mg
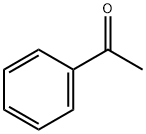
98-86-2
799 suppliers
$9.00/5g

85658-55-5
44 suppliers
$45.00/25mg