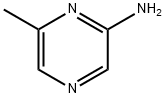
2-Amino-6-methylpyrazine synthesis
- Product Name:2-Amino-6-methylpyrazine
- CAS Number:5521-56-2
- Molecular formula:C5H7N3
- Molecular Weight:109.13
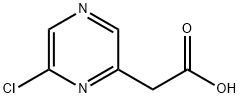
930798-25-7

5521-56-2
1. A solution of diethyl malonate (2.1 mmol) in THF (60 ml) was mixed with a solution of 2,6-dichloropyrazine (20 g) in THF (40 ml) at 60 °C. 2. The mixture was heated to reflux for 18 hrs and subsequently cooled to room temperature and 2M hydrochloric acid (100 ml) was added. 3. The two layers of the reaction solution were separated and the THF layer was partially concentrated under vacuum to give a solution containing diethyl 2-(6-chloropyrazin-2-yl)malonate. 4. The above solution was cooled to 10 °C, 2 M sodium hydroxide (328 ml) was added and stirred for 24 hours. 5. The mixture was washed with methyl isobutyl ketone (MIBK, 200 ml) and the organic layer was discarded. 6. The aqueous layer containing 2-(6-chloropyrazin-2-yl)malonic acid was added to 6M hydrochloric acid (135 ml) and the reaction temperature was controlled at 20-25°C to promote the decarboxylation reaction. 7. 6-Chloropyrazin-2-ylacetic acid was partially precipitated and extracted into MIBK (130 ml) for separation, dried with MgSO4, filtered and evaporated to give a yellow solid. 8. The crude product (22.4 g) was crystallized from methyl tert-butyl ether (MTBE) to give pure 6-chloropyrazin-2-ylacetic acid in 67% yield (15.4 g) based on 2,6-dichloropyrazine. 9. 6-Chloropyrazin-2-ylacetic acid (20.0 g) was reacted with ammonia (120 ml) in a sealed vessel at 180 °C (35 bar) for 8 hours. 10. The reaction mixture was cooled to 20 °C, water (40 ml) was added and the ammonia was removed by vacuum concentration. 11. The product was extracted into ethyl acetate, the solution was treated with charcoal and subsequently dried over MgSO4, filtered and evaporated to give 6-methyl-2-pyrazinamine as a light green solid (9.0 g, 71% yield based on 6-chloropyrazin-2-ylacetic acid). Analytical Data: - Diethyl 2-(6-chloropyrazin-2-yl)malonate: MS (EI+ve) 273/275 (M+H); 1H NMR (CDCl3): δ 8.7 (1H, s), 8.5 (1H, s), 4.9 (1H, s), 4.2 (4H, q), 1.2 (6H, t). - 6-Chloropyrazin-2-ylacetic acid: MS (EI+ve) 173/175 (M+H); 1H NMR (CDCl3): δ 8.55 (1H, s), 8.50 (1H, s); 3.9 (2H, s). - 6-Methyl-2-pyrazinamine: MS (GC/MS): 100% RT 2.0 min, M+H = 110; 1H NMR (CDCl3): δ 7.8 (1H, s), 7.8 (1H, s), 4.4 (2H, bs), 2.4 (3H, s). Mpt: 124-125°C.

930798-25-7
86 suppliers
$72.00/250mg

5521-56-2
138 suppliers
$33.00/200mg
Yield: 71%
Reaction Conditions:
with ammonia in water at 180; under 26252.6 Torr; for 8 h;sealed vessel;
Steps:
1.c
60ml) at O0C was added, diethylmalonate (2.1moleq) in THF (60ml) and 2,6- dichloropyrazine (2Og) in THF (40ml). The mixture was then heated to reflux for 18hrs before being allowed to cool and 2M Hydrochloric acid (100ml) added. The resulting two layers were separated and the THF layer partially concentrated under vacuum to give a solution containing 2-(6-chloro-pyrazin-2-yl)-malonic acid diethyl ester. This solution was EPO
References:
ASTRAZENECA AB WO2007/35153, 2007, A1 Location in patent:Page/Page column 2; 4-5
544-97-8
129 suppliers
$130.00/5g
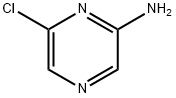
33332-28-4
299 suppliers
$9.00/1g

5521-56-2
138 suppliers
$33.00/200mg
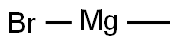
75-16-1
275 suppliers
$12.00/10ml

33332-28-4
299 suppliers
$9.00/1g

5521-56-2
138 suppliers
$33.00/200mg
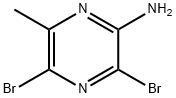
74290-66-7
73 suppliers
$76.00/250mg

5521-56-2
138 suppliers
$33.00/200mg
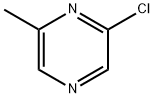
38557-71-0
177 suppliers
$5.00/250mg

5521-56-2
138 suppliers
$33.00/200mg