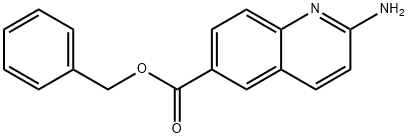
2-Aminoquinoline-6-carboxylic acid benzyl ester synthesis
- Product Name:2-Aminoquinoline-6-carboxylic acid benzyl ester
- CAS Number:863492-35-7
- Molecular formula:C17H14N2O2
- Molecular Weight:278.31
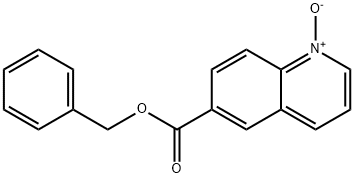
863492-34-6
1 suppliers
inquiry

863492-35-7
26 suppliers
inquiry
Yield:863492-35-7 55%
Reaction Conditions:
with ammonium chloride;triethylamine;p-toluenesulfonyl chloride in 1,2-dichloro-ethane at -10 - 25; for 9 - 20 h;Product distribution / selectivity;
Steps:
b
(b) Preparation of Intermediate 2-Amino-auinoline-6, carboxvlic acid benzvl ester; To a solution of 1-oxy-quinoline-6-carboxylic acid benzyl ester (114.29 g, 0.409 moles) in 1, 2-dichloroethane (1,368 mL) was added p-toluenesulfonyl chloride (109.24 g, 0.573 moles). The reaction mixture was stirred for one to four hours at 20°C to 25°C. In a separate reactor, triethylamine (135.04 g, 1.334 moles) was added to a slurry of ammonium chloride (65.63 g, 1.227 moles) in 1, 2-dichloroethane (342 mL). The ammonium chloride slurry was then cooled to-5°C to-10°C. The p-toluenesulfonyl chloride solution was then added over three to four hours to the ammonium chloride slurry which was maintained at -10°C to -5°C. The reaction mixture was stirred for 8 to 16 hours at-10°C to-5°C, and product obtained by filtration. The product was then slurried in water (1,150 mL) for 8 to 16 hours at 20°C to 25°C and isolated by filtration (62.33 g, 55% yield).
References:
WO2005/80373,2005,A1 Location in patent:Page/Page column 83
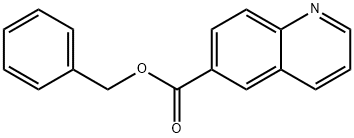
863492-33-5
1 suppliers
inquiry

863492-35-7
26 suppliers
inquiry
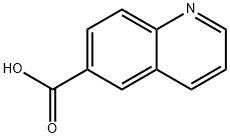
10349-57-2
317 suppliers
$10.00/1g

863492-35-7
26 suppliers
inquiry

100-51-6
1435 suppliers
$5.00/100g

863492-35-7
26 suppliers
inquiry