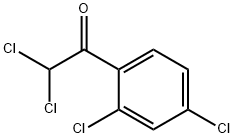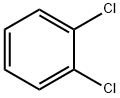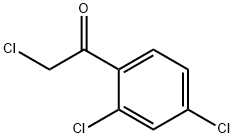
2,2',4'-Trichloroacetophenone synthesis
- Product Name:2,2',4'-Trichloroacetophenone
- CAS Number:4252-78-2
- Molecular formula:C8H5Cl3O
- Molecular Weight:223.48
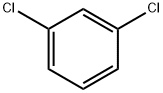
2,2',4'-Trichloroacetophenone is a reagent used in the synthesis of isoconazole, a potent antifungal agent. It was prepared from m-dichlorobenzene and alchlor by stirring with Chloracetyl chloride under 40-50 °C for 9 h.
Yield:4252-78-2 93.1%
Reaction Conditions:
with aluminum (III) chloride at 30; for 3 h;Friedel-Crafts Acylation;
Steps:
4.4. General procedure for the synthesis of compounds 8a-8b
General procedure: To a mixture of m-dichlorobenzene (6a) (14.6 g, 0.1 mol) andanhydrous aluminum trichloride (21.3 g, 0.16 mol) was added 2-chloroacetyl chloride (7) (12.4 g, 0.11 mol) dropwise at room temperatureand the dropping rate needed to be controlled so that thereaction temperature didn’t exceed 30 °C. The mixture was stirredat 30 °C for 3 h. Then, the solution was carefully added to themixture of ice and water (100 g) which contained concentratedhydrochloric acid (5 mL). The mixture was stirred to form a darkbrown oil, then was extracted with dichloromethane (3 x 40 mL).The combined organic layers were washed with brine (40 mL),dried over anhydrous sodium carbonate overnight. The desiccantwas filtered off and yellow solid was obtained under reducedpressure. The solid was purified by recrystallization with ethanol togive a white solid (6a). Yield 93.1%, LC-MS m/z: 222.9 [M+H]+.According to the preparation method of 8a, m-difluorobenzenewasused as raw material to prepare the intermediate 8b: white solid, Yield 92.4%, LC-MS m/z: 191.01 [M+H]+.
References:
An, Ran;Guo, Chun;Guo, Meng-bi;Hou, Zhuang;Mou, Yan-hua;Su, Xin;Xu, Hang [European Journal of Medicinal Chemistry,2020,vol. 198]
1475-13-4
73 suppliers
$13.00/1g

4252-78-2
375 suppliers
$9.00/25g