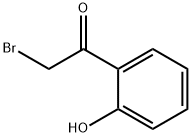
2-Bromo-2′-hydroxyacetophenone synthesis
- Product Name:2-Bromo-2′-hydroxyacetophenone
- CAS Number:2491-36-3
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
118-93-4

2491-36-3
The general procedure for the synthesis of 2-bromo-1-(2-hydroxyphenyl)ethanone from 2'-hydroxyacetophenone is as follows: in a 50 mL round-bottomed flask equipped with a magnetic stirrer, 2'-hydroxyacetophenone (10 mmol) was added. Subsequently, potassium bromide (40 mmol) dissolved in 20 mL of water was added to this solution and mixed with stirring at room temperature. Next, 30% hydrogen peroxide (4 mmol) was slowly added. Vanadium (V) complex (0.01 mmol) and 70% perchloric acid (4 mmol) were further added to the reaction system, and the reaction mixture was stirred at 0°C, followed by raising to room temperature and continued stirring for 10 minutes. Under continuous stirring conditions, additional 70% perchloric acid (4 mmol) was added every 10 min for three times. The reaction process was monitored by thin layer chromatography (TLC). The nuclear magnetic resonance (NMR) spectral data of the final brominated product is detailed in the Supporting Information.
582-24-1
253 suppliers
$8.00/5g

2491-36-3
134 suppliers
$10.00/1g
Yield: 60%
Reaction Conditions:
with bromine;acetic acid for 2 h;Reflux;
Steps:
Synthesis of 3-bromoacetyl-4-hydroxy-6-methyl-2H-pyran-2-one 2c:
General procedure: A solution of bromine (0.27 mL, 5 mmol) inacetic acid (10 mL) is added stepwise to a solution of DHA 1 (0.84 g, 5 mmol) in acetic acid (20 mL). After a reflux of 2h, the reaction mixture was poured into water (100 mL) and ice (50 g). The obtained solid was filtered-off andrecrystallized from a 1:1 mixture of hexane-chloroform, being compound 2c obtained as yellow crystals (0.75 g, 61%):mp 117°C (mp 118-119 ).
References:
Lechani, Nawel;Hamdi, Maamar;Kheddis-Boutemeur, Baya;Talhi, Oualid;Laichi, Yacine;Bachari, Khaldoun;Silva, Artur M. S. [Synlett,2018,vol. 29,# 11,p. 1502 - 1504] Location in patent:supporting information

118-93-4
568 suppliers
$10.00/25g

2491-36-3
134 suppliers
$10.00/1g
![Ethanone, 1-[2-(acetyloxy)phenyl]-2-bromo-](/CAS/20210111/GIF/40231-08-1.gif)
40231-08-1
0 suppliers
inquiry

2491-36-3
134 suppliers
$10.00/1g
620-72-4
201 suppliers
$26.00/5g

2491-36-3
134 suppliers
$10.00/1g
108-95-2
772 suppliers
$14.00/25g

2491-36-3
134 suppliers
$10.00/1g