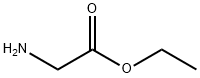
ethyl glycinate synthesis
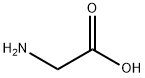
- Product Name:ethyl glycinate
- CAS Number:459-73-4
- Molecular formula:C4H9NO2
- Molecular Weight:103.12
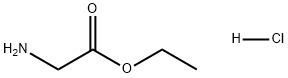
623-33-6
657 suppliers
$5.00/10g

459-73-4
56 suppliers
inquiry
Yield:459-73-4 96%
Reaction Conditions:
with triethylamine in chloroform at 20; for 7 h;Reflux;
Steps:
4.2.2. Synthesis of ethyl 2-aminoacylates
General procedure: 4.2.2.1. General synthetic procedure. To a suspension of amino acidethyl ester hydrochloride (71.6 mmol) in chloroform (15 ml) wasadded dropwise a solution of triethylamine (71.6 mmol) in chloroform(10 ml) at room temperature. The resulting reaction mixture was stirred at the same temperature for 6 h, followed by heating atreflux for 1 h. The mixturewas allowed to cool to room temperatureand solvent was removed under reduced pressure to afford a crudematerial. The product was extracted with diethyl ether (3 25 ml)and then filtered. The filtrates were washed with ether (2 10 ml).The filtered solution was concentrated in vacuo to afford pure ethyl-2-aminoacylates.4.2.2.2. Ethyl 2-aminoacetate 12. Physical characteristics: yellowoil; Yield: 7.09 g, 96%; 1H NMR (CDCl3) d 4.14 (2H, q, J 7.2 Hz,OCH2CH3), 3.40 (2H, s, CH2CO), 1.67 (2H, s, NH2), 1.23 (3H, t,J 7.2 Hz, OCH2CH3); 13C NMR (CDCl3) d 174.2 (C]O), 60.8(OCH2CH3) 43.9 (CH2CO), 14.2 (OCH2CH3).
References:
Bode, Moira L.;Coyanis, E. Mabel;Mosebi, Salerwe;Njengele, Zikhona;Rashamuse, Thompho J.;Sayed, Yasien [European Journal of Medicinal Chemistry,2020,vol. 190]
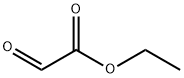
924-44-7
271 suppliers
$12.00/5g

459-73-4
56 suppliers
inquiry
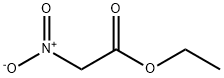
626-35-7
267 suppliers
$5.00/1g

459-73-4
56 suppliers
inquiry