Ethyl nitroacetate synthesis
- Product Name:Ethyl nitroacetate
- CAS Number:626-35-7
- Molecular formula:C4H7NO4
- Molecular Weight:133.1
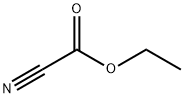
623-49-4
244 suppliers
$10.60/1gm:

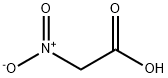
626-35-7
263 suppliers
$5.00/1g
Yield:626-35-7 83%
Reaction Conditions:
with acetic acid in nitromethane;dimethyl sulfoxide
Steps:
7 EXAMPLE 7
EXAMPLE 7 To a 100 ml three-neck round bottom flask, equipped with an addition funnel, a thermometer and a condenser topped with a nitrogen gas inlet tube, was added 20 ml of dimethylsulfoxide, 0.196 g (0.00815 mole) sodium hydride and then 0.5 g (0.00815 mole) of nitromethane was added dropwise at ambient temperature to give a slurry. 1.6 ml (0.0163 mole) of ethyl cyanoformate was then added dropwise forming a homogeneous solution and was then stirred for 2 hours at ambient temperature. The solution was made acidic by adding 30 ml of 1 Normal acetic acid with stirring. The product was extracted with ethyl acetate and dried over magnesium sulfate. Ethyl nitroacetate was formed in 83% yield as determined by gas chromatography.
References:
W. R. Grace & Co.-Conn. US4873358, 1989, A
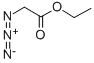
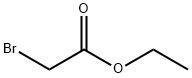
637-81-0
181 suppliers
$40.00/500mg

626-35-7
263 suppliers
$5.00/1g
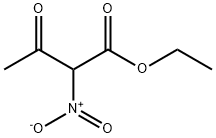
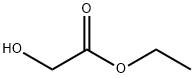
51026-98-3
9 suppliers
inquiry

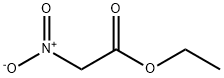
626-35-7
263 suppliers
$5.00/1g