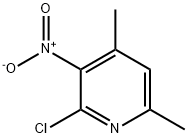
2-Chloro-4,6-diMethyl-3-nitropyridine synthesis
- Product Name:2-Chloro-4,6-diMethyl-3-nitropyridine
- CAS Number:89793-09-9
- Molecular formula:C7H7ClN2O2
- Molecular Weight:186.6

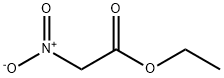
22934-13-0

89793-09-9
The reaction was carried out at 100 °C for 5 h with stirring, using 4,6-dimethyl-3-nitropyridin-2(1H)-one (9.0 g, 53.5 mmol) as raw material, which was dissolved in phosphorous trichloride (50 mL). Upon completion of the reaction, the reaction solution was cooled to room temperature and subsequently concentrated under reduced pressure to remove the solvent. The resulting residue was dissolved in dichloromethane (50 mL) and the pH was adjusted to >7 by slow dropwise addition of saturated sodium bicarbonate solution at 0 °C. The organic phase was separated, dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure and ground with petroleum ether to give 2-chloro-4,6-dimethyl-3-nitropyridine (9.0 g, 90% yield) as a brown solid. lc-MS m/z: 187.1 [M + H]+; purity (214 nm): 96%; tR = 1.76 min.

22934-13-0
25 suppliers
inquiry

89793-09-9
36 suppliers
inquiry
Yield:89793-09-9 90%
Reaction Conditions:
with trichlorophosphate at 95; for 3 h;
Steps:
1.2
A mixture of 4,6-dimethyl-3-nitro-2(1 H)-pyridinone (step 1 , 10.0 g, 29.7 mmol) in phosphorus oxychloride (35 mL, 187.3 mmol) was stirred at 95 °C for 3 h, then cooled to 45 °C. The excess amount of phosphorus oxychloride was removed by distillation under reduced pressure at 45 °C. The residue was cooled to room temperature, and diluted with dichloromethane (75 mL). The resulting solution was cooled to 0°C, and 2N hydrochloric acid (50 mL) was added dropwise into the solution. The organic layer was separated, and washed with 2N hydrochloric acid (4 x 25 mL), 2N aqueous NaOH (2 x 50 mL) and brine (50 mL). The organic phase was dried (MgSO4) and concentrated under reduced pressure to give 10.0 g (90%) of the title compound as white solids: 1H-NMR (CDCI3) δ 7.07 (1 H, s), 2.56 (3H1 s), 2.35 (3H, s).
References:
WO2006/95268,2006,A1 Location in patent:Page/Page column 28
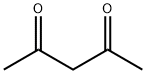
123-54-6
588 suppliers
$10.00/25ml

89793-09-9
36 suppliers
inquiry

22934-13-0
25 suppliers
inquiry

89793-09-9
36 suppliers
inquiry