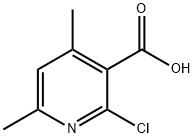
2-Chloro-4,6-dimethyl-3-pyridinecarboxylic acid synthesis
- Product Name:2-Chloro-4,6-dimethyl-3-pyridinecarboxylic acid
- CAS Number:66662-48-4
- Molecular formula:C8H8ClNO2
- Molecular Weight:185.61
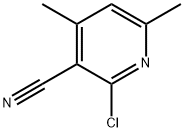
14237-71-9

66662-48-4
Example C Synthesis of 2-chloro-4,6-dimethylnicotinic acid: 15 g of 2-chloro-3-cyano-4,6-dimethylpyridine [Jahine, J. prakt. Chem. 316, 337 (1974)] was mixed with 40 ml of concentrated sulphuric acid and 13 ml of fuming nitric acid, and heated with stirring to 95° C to initiate an exothermic reaction. The reaction temperature was maintained between 95 °C and 100 °C by cooling with ice water. After the exothermic reaction subsided, stirring was continued at 100°C for 30 minutes. Subsequently, the reaction mixture was decanted into ice and the pH was adjusted with ammonia to 3-4. The precipitate was collected by filtration and washed with water. The resulting solid was dehydrated by azeotropy with toluene in a device fitted with a water trap and finally recrystallized by toluene to give the target compound in 80% yield of the theoretical value with a melting point of 159-160°C. The results of elemental analysis were as follows: molecular formula C8H8ClNO2, molecular weight 185.6; calculated values: C 51.77%, H 4.34%, Cl 19.10%, N 7.55%; measured values: C 51.85%, H 4.36%, Cl 19.25%, N 7.56%.

14237-71-9
190 suppliers
$6.00/250mg

66662-48-4
76 suppliers
$18.00/250mg
Yield:66662-48-4 80%
Reaction Conditions:
with sulfuric acid;nitric acid in water;toluene;
Steps:
C 2-Chloro-4,6-dimethyl-nicotinic acid
EXAMPLE C 2-Chloro-4,6-dimethyl-nicotinic acid A mixture consisting of 15 gm of 2-chloro-3-cyano-4,6-dimethyl-pyridine [see Jahine, J. prakt. Chem. 316,337 (1974)], 40 ml of concentrated sulfuric acid and 13 ml of fuming nitric acid was heated while stirring. At 95° C. an exothermic reaction took place. By cooling with ice water the temperature was maintained at 95°-100° C. After the exothermic reaction had subsided, the mixture was stirred for 30 minutes more at 100° C. and then poured on ice and adjusted to a pH-value of 3-4 with ammonia. The resulting precipitate was collected by suction filtration, washed with water, dehydrated by boiling with toluene in a vessel equipped with a water trap, and recrystallized from toluene, yielding 80% of theory of the compound named in the heading, which had a melting point of 159°-160° C. Elemental analysis: C8 H8 ClNO2; mol. wt. 185.6; Calculated: C-51.77% H-4.34%; Cl-19.10%; N-7.55%; Found: C-51.85% H-4.36%; Cl-19.25%; N-7.56%.
References:
US4167570,1979,A
140413-44-1
59 suppliers
$45.00/50mg

66662-48-4
76 suppliers
$18.00/250mg
769-28-8
254 suppliers
$5.00/1g

66662-48-4
76 suppliers
$18.00/250mg