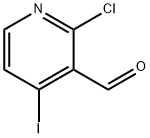
2-Chloro-4-iodopyridine-3-carboxaldehyde synthesis
- Product Name:2-Chloro-4-iodopyridine-3-carboxaldehyde
- CAS Number:153034-90-3
- Molecular formula:C6H3ClINO
- Molecular Weight:267.45
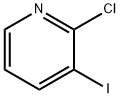
78607-36-0
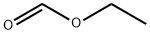
109-94-4

153034-90-3
Example 1: Synthesis of N-(2-(2,6-dichlorophenyl)-2H-pyrazolo[4,3-c]pyridin-4-yl)cyclopropanecarboxamide Step 1: Preparation of 2-chloro-4-iodonicotinaldehyde A solution of 2-chloro-3-iodopyridine (5.0 g, 21 mmol) in anhydrous THF (30 mL) was slowly added dropwise to a solution of lithium diisopropylammonium (15 mL, 30 mmol) in anhydrous THF (50 mL) that was pre-cooled to -78 °C. The reaction mixture was stirred continuously at -78 °C for 3 hours. Subsequently, ethyl formate (4.0 g, 54 mmol) was added to the reaction system and stirring was continued at the same temperature for 1.5 hours. Upon completion of the reaction, the reaction was quenched by the addition of water (10 mL), after which the mixture was slowly warmed to room temperature. THF was removed by distillation under reduced pressure after addition of 2 M HCl (50 mL).The aqueous phase was extracted with ethyl acetate (2 x 50 mL). The organic phases were combined, washed with brine, dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: ether/petroleum ether=1:4) to afford the target compound 2-chloro-4-iodonicotinaldehyde as a yellow solid (3.0 g, 54% yield). NMR (500MHz, CDCl3): δ10.22 (s, 1H), 8.09 (d, J=5.0Hz, 1H), 7.95 (d, J=5.0Hz, 1H).
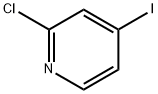
153034-86-7
384 suppliers
$10.00/1g
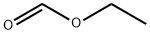
109-94-4
506 suppliers
$10.00/10g

153034-90-3
194 suppliers
$10.00/250mg
Yield:153034-90-3 54%
Reaction Conditions:
Stage #1: 2-chloro-4-iodopyridinewith lithium diisopropyl amide in tetrahydrofuran at -78; for 3 h;
Stage #2: formic acid ethyl ester in tetrahydrofuran at -78; for 1.5 h;
Steps:
Step 1:2-Chloro-4-iodonicotinaldehyde
A mixture of 2-chloro-3-iodopyridine(5.0g, 21mmol) in dry THF (30 mL) was slowly added to a cold (-78°C) solution oflithiumdiisopropylamide(15 mL, 30mmol) in dry THF (50 mL). The resulting mixture was stirred for 3h at this temperature.Ethylformate(4.0g, 54mmol) wasthenadded.Stirring continued for 1.5 h at the same temperature.Water (10 mL) was added to quench the reaction, and then theresultingmixture was warmed toroom temperature.2 MHCl(50 mL) was added and then the THF was removed under reduced pressure.The aqueous residue was extracted withethyl acetate(2 x 50 mL).The combined organic extracts were washed with brine, dried over Na2SO4andconcentrated under reduced pressure.The residuewas purified on silica gel(diethyl ether/petroleum ether1:4)to give the desired product as a yellow solid(3.0 g, 54% yield).HNMR(500 MHz, CDCl3):δ10.22(s, 1H), 8.09(d, J =5.0Hz, 1H), 7.95(d,J =5.0Hz, 1H).
References:
Liang, Jun;Van Abbema, Anne;Balazs, Mercedesz;Barrett, Kathy;Berezhkovsky, Leo;Blair, Wade S.;Chang, Christine;Delarosa, Donnie;DeVoss, Jason;Driscoll, Jim;Eigenbrot, Charles;Goodacre, Simon;Ghilardi, Nico;MacLeod, Calum;Johnson, Adam;Bir Kohli, Pawan;Lai, Yingjie;Lin, Zhonghua;Mantik, Priscilla;Menghrajani, Kapil;Nguyen, Hieu;Peng, Ivan;Sambrone, Amy;Shia, Steven;Smith, Jan;Sohn, Sue;Tsui, Vickie;Ultsch, Mark;Williams, Karen;Wu, Lawren C.;Yang, Wenqian;Zhang, Birong;Magnuson, Steven [Bioorganic and Medicinal Chemistry Letters,2017,vol. 27,# 18,p. 4370 - 4376] Location in patent:supporting information

78607-36-0
370 suppliers
$14.00/1g
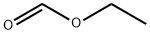
109-94-4
506 suppliers
$10.00/10g

153034-90-3
194 suppliers
$10.00/250mg