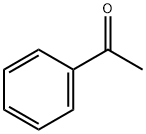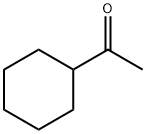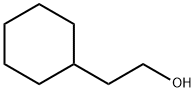
2-Cyclohexylethanol synthesis
- Product Name:2-Cyclohexylethanol
- CAS Number:4442-79-9
- Molecular formula:C8H16O
- Molecular Weight:128.21
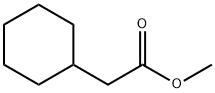
14352-61-5
78 suppliers
$20.00/1g

4442-79-9
189 suppliers
$10.00/1g
Yield:4442-79-9 99%
Reaction Conditions:
with [RuCl2((E)-N-(2-(diphenylphosphino)benzyl)-1-(6-((diphenylphosphino)methyl)pyridin-2-yl)methanimine)];hydrogen;sodium ethanolate at 80; under 37503.8 Torr; for 4 h;Autoclave;
Steps:
10 General hydrogenation reaction procedure:
General procedure: Ester, ruthenium catalyst , metal alkoxide co-catalyst (used as a solid or some alcoholic solution) and optionally solvent (see Table 1) were loaded altogether in an 100 mL or 1L autoclave equipped with a mechanical stirring device, pressure and internal temperature sensors and a heating/cooling system for internal temperature regulation. Sealed autoclave was then purged under stirring with nitrogen (3 times 5 bars) and hydrogen (3 times 5 bars) before being pressurized to required hydrogen pressure via an hydrogen tank equipped with a way out pressure regulator and also an internal pressure sensor to follow and determine hydrogen consumption. Reaction mixture was then heated to required temperature and hydrogen pressure into the autoclave was maintained to the desired value during the whole reaction. Upon reaction completion also determined by GC analysis with complete disappearance of both starting material and mixed ester coming from transesterification reaction with product and eventually with metal alkoxide co-catalyst and/or alcoholic solvent, autoclave was then cooled down to 25 °C. It was then depressurized and purged with nitrogen (3 times 5 bars) and reaction mixture was then transferred to a round-bottomed flask and lights compounds were removed under vacuum. Crude product was then flash distilled in order to determine the quantity of residues formed during the reaction and yield was calculated based on GC purity of distilled product.
References:
FIRMENICH SA;DUPAU, Philippe;BONOMO, Lucia;KERMORVAN, Laurent;HALDIMANN SANCHEZ, Murielle WO2019/175158, 2019, A1 Location in patent:Page/Page column 27; 35
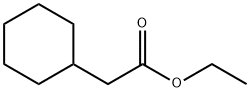
5452-75-5
79 suppliers
$37.00/25mL

4442-79-9
189 suppliers
$10.00/1g
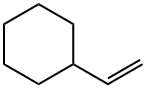
695-12-5
105 suppliers
$53.00/5mL

4442-79-9
189 suppliers
$10.00/1g
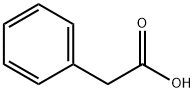
103-82-2
0 suppliers
$22.19/5g

4442-79-9
189 suppliers
$10.00/1g
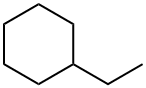
1678-91-7
205 suppliers
$36.00/25mL