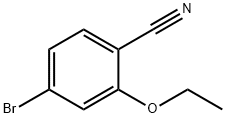
2-ethyoxyl-4-broMobenzonitrile synthesis
- Product Name:2-ethyoxyl-4-broMobenzonitrile
- CAS Number:1255870-63-3
- Molecular formula:C9H8BrNO
- Molecular Weight:226.07

75-03-6
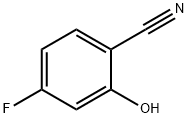
186590-01-2

1255870-63-3
General procedure for the synthesis of 2-ethoxy-4-bromobenzonitrile from 4-fluoro-2-hydroxybenzonitrile and iodoethane: 4-fluoro-2-hydroxybenzonitrile (50 mg, 0.32 mmol) was dissolved in N,N-dimethylformamide (DMF, 2 mL) in a round-bottomed flask and finely ground potassium carbonate (133 mg, 0.96 mmol) was added under stirring conditions. Subsequently, ethidium iodide (0.03 mL, 0.39 mmol) was slowly added dropwise to the reaction mixture. The reaction process was monitored by thin layer chromatography (TLC) until the feedstock completely disappeared. Upon completion of the reaction, the DMF solvent was removed by distillation under reduced pressure. The residue was dissolved in ethyl acetate and subsequently washed with saturated saline. The organic phase was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with the eluent ratio of petroleum ether: ethyl acetate = 5:1 to give 2-ethoxy-4-bromobenzonitrile (53.8 mg, 94% yield).

75-03-6
365 suppliers
$11.00/5g

186590-01-2
64 suppliers
$14.00/1g

1255870-63-3
40 suppliers
inquiry
Yield:1255870-63-3 94%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide;
Steps:
43 (Experimental Method 1) 2-Ethoxy-4-bromo-benzonitrile
4-fluoro-2-hydroxybenzonitrile (50 mg, 0.32 mmol) and DMF (2 ml) were put in a round-bottom flask and stirred, finely ground potassium carbonate (133 mg, 0.96 mmol) was added thereto, and then iodoethane (0.03 ml, 0.39 mmol) was added thereto, followed by stirring.
After confirming by TLC that the base compound had disappeared, DMF was vacuum-distilled, and the residue was diluted with ethyl acetate and then washed with brine.
The residue obtained after vacuum distillation was subjected to column chromatography (hexane:ethyl acetate=5:1) to obtain 2-ethoxy-4-bromo-benzonitrile (53.8 mg).
(Yield: 94%)
References:
US2018/265462,2018,A1 Location in patent:Paragraph 0151

75-03-6
365 suppliers
$11.00/5g
288067-35-6
165 suppliers
$5.00/250mg

1255870-63-3
40 suppliers
inquiry

64-17-5
814 suppliers
$10.00/50g
105942-08-3
382 suppliers
$7.00/5g

1255870-63-3
40 suppliers
inquiry