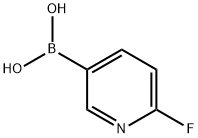
2-Fluoropyridine-5-boronic acid synthesis
- Product Name:2-Fluoropyridine-5-boronic acid
- CAS Number:351019-18-6
- Molecular formula:C5H5BFNO2
- Molecular Weight:140.91
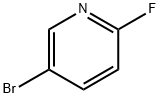
766-11-0

351019-18-6
The general procedure for the synthesis of 2-fluoropyridine-5-boronic acid from 2-fluoro-5-bromopyridine is as follows: 1. in a 72 L reactor equipped with a reflux condenser and temperature probe, 5-bromo-2-fluoropyridine (1.17 L, 0.568 mol), toluene (18.2 L) and triisopropyl borate (3.13 L, 0.68 mol, 1.2 eq.) were added and stirring was initiated. 2. tetrahydrofuran (4.4L) was added to the reactor and the reaction mixture was cooled to -35 to -50°C. 3. n-Butyllithium (2.5M hexane solution, 5.44L, 0.68mol, 1.2 eq.) was added slowly and dropwise while maintaining the temperature of the reaction mixture between -35 and -45 °C. 4. after 5 hours of reaction, confirming that the reaction is complete, the reaction mixture is slowly warmed to -15 to -20 °C. 5. 2M HCl (11.80L) was slowly added to the reaction mixture while maintaining the temperature between -15°C and 0°C. 6. The reaction mixture was stirred at 18 to 23 °C for 16 h followed by phase separation. 7. The organic phase was extracted with 6 M sodium hydroxide (6.0 L). 8. The acidic and basic aqueous phases were combined in a reactor and the pH was adjusted to 7.5 by slowly adding 6M HCl (2.5L). 9. Sodium chloride (6.0 kg) was added to the aqueous phase, followed by extraction with THF (3 x 20 L). 10. The organic phases were combined, dried with magnesium sulfate, and concentrated to give 1300 g of brown solid in 81% crude yield.

766-11-0
452 suppliers
$6.00/5g

351019-18-6
324 suppliers
$11.19/1G
Yield:351019-18-6 81%
Reaction Conditions:
Stage #1:5-bromo-2-fluoropyridine with Triisopropyl borate in tetrahydrofuran;toluene at -50 - -35;
Stage #2: with n-butyllithium at -45 - -15; for 5 h;Product distribution / selectivity;
Steps:
3
Preparation of 6-fluoropyridin-3-ylboronic acid (4); [000254] A 72 L reactor equipped with reflux condenser, and temperature probe. To the reactor 5-bromo-2-fluoropyridine (1.17 L, 0.568 mol), toluene (18.2 L), and triisopropyl borate (3.13 L, 0.68 mol, 1.2 equiv.) were charged and stirred. Tetrahydrofuran (4.4 L) was added to the reactor and the reaction mixture was cooled to between -35 to -50 0C. While maintaining a temperature between -35 to -45 0C, n-butyl lithium (2.5 M solution of hexanes, 5.44 L, 0.68 mol, 1.2 equiv.) was cautiously added to the reactor. After 5 h, the reaction was deemed complete and the reaction mixture was warmed to between -15 to -20 0C. To the reaction was added 2M HCl (11.80L) to the reactor while maintaining a temperature between -15 0C and 0 0C. The reaction mixture was stirred at 18 to 23 0C for (16 h) and the phases were separated. The organics were then extracted with 6 M sodium hydroxide (6.0 L). The acidic anbasic aqueous phases were mixed in the reactor and 6 M HCl (2.5 L) was added until pH 7.5 was achieved. Sodium chloride (6.0 kg) was then added to the aqueous phase. The aqueous phase was then extracted with THF (3 * 20 L). The combined organics were dried with magnesium sulfate and concentrated to give 1300 g of a tan solid (81% crude yield).
References:
KINEX PHARMACEUTICALS, LLC WO2009/51848, 2009, A1 Location in patent:Page/Page column 65

766-11-0
452 suppliers
$6.00/5g

121-43-7
381 suppliers
$13.00/25mL

351019-18-6
324 suppliers
$11.19/1G
171197-80-1
299 suppliers
$20.00/1g

351019-18-6
324 suppliers
$11.19/1G
20511-12-0
461 suppliers
$6.00/1g

351019-18-6
324 suppliers
$11.19/1G