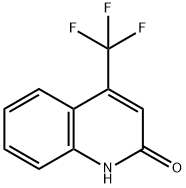
2-Hydroxy-4-(trifluoromethyl)quinoline synthesis
- Product Name:2-Hydroxy-4-(trifluoromethyl)quinoline
- CAS Number:25199-84-2
- Molecular formula:C10H6F3NO
- Molecular Weight:213.16
Experimental Procedure: In a 1 L round flask fitted with a reflux condenser a mixture of 25.60 g aniline, 50.0 g ethyl-4,4,4-trifluoroacetoacetate 300 ml toluene was heated to reflux in an oil bath at 130 °C. After 20 min 3 ml water was added and the mixture was heated at reflux for another 24 h. the reaction mixture was cooled to room temperature and concentrated under reduced pressure. A round flask with 200 mL H2SO4 was heated to 80°C and the crude oil from the step before was added in portions to the H2SO4 keeping the internal temperature below 90°C, total addition time was approximately 40 min. After addition was complete, the mixture was stirred at 80°C for 1 h, cooled and poured onto 400 g crushed ice. The resulting solids were filtered, washed with water, and dried under vacuum at 40°C to give 33.0 g (50%) product (4-Trifluoromethylquinolin-2(1H)-one) as a colorless solid.

62-53-3
688 suppliers
$10.00/1g
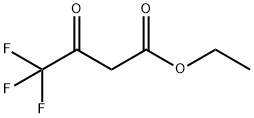
372-31-6
488 suppliers
$5.00/10g

25199-84-2
126 suppliers
$34.00/250mg
Yield: 73%
Reaction Conditions:
Stage #1:aniline;ethyl 4,4,4-trifluoroacetoacetate with triethylamine in toluene for 7 h;Heating;
Stage #2: with sulfuric acid at 90;Knorr-Conrad-Limpach cyclization;
References:
Berbasov, Dmitrii O.;Soloshonok, Vadim A. [Synthesis,2003,# 13,p. 2005 - 2010]
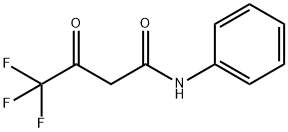
836-31-7
9 suppliers
inquiry

25199-84-2
126 suppliers
$34.00/250mg