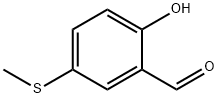
2-hydroxy-5-(methylthio)benzaldehyde synthesis
- Product Name:2-hydroxy-5-(methylthio)benzaldehyde
- CAS Number:67868-84-2
- Molecular formula:C8H8O2S
- Molecular Weight:168.21

50-00-0
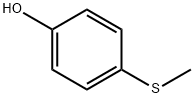
1073-72-9

67868-84-2
To a suspension of 4-(methylthio)phenol (50 g, 357 mmol), paraformaldehyde (72.3 g, 2407 mmol) and anhydrous magnesium chloride (50.9 g, 535 mmol) in acetonitrile (500 mL) was added triethylamine (186 mL, 1337 mmol). The reaction mixture was stirred at room temperature and then warmed to 60 °C and stirred continuously for 5 hours. Upon completion of the reaction, the mixture was cooled to 0 °C and 1N hydrochloric acid was slowly added until the two phases separated (~1.5 L). Methyl tert-butyl ether (MTBE, 700 mL) was then added and the organic layer was separated. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by fast column chromatography on silica gel with the eluent being a 1:1 hexane/dichloromethane solvent mixture to afford 2-hydroxy-5-(methylthio)benzaldehyde (50.5 g, 300 mmol, 84% yield) as a semi-solid.

50-00-0
896 suppliers
$10.00/25g

1073-72-9
227 suppliers
$6.00/1g

67868-84-2
55 suppliers
$18.00/100mg
Yield:67868-84-2 84%
Reaction Conditions:
with triethylamine;magnesium chloride in acetonitrile at 60; for 5 h;
Steps:
A Step A: Preparation of 2-hvdroxy-5-(methylthio)benzaldehvde:
To a suspension of 4-methylsulfanylphenol (50 g, 357 mmol), paraformaldehyde (72.3 g, 2407 mmol), and anhydrous magnesium chloride (50.9 g, 535 mmol) in acetonitrile (500 mL) was added triethyl amine (186 mL, 1337 mmol) at ambient temperature. After the addition, the reaction mixture was stirred at 60 °C for 5 hours. After cooling to 0 °C, 1 N HC1 was added slowly until two phase separated (ca. 1.5 L). MTBE (700 mL) was added. The organic layer was separated, washed with brine, dried (sodium sulfate), filtered and concentrated under reduced pressure. The residue obtained was purified by flash chromatography on silica gel eluting with 1 : 1 hexane/dichloromethane to give 2-hydroxy-5-methylsulfanyl-benzaldehyde (50.5 g, 300 mmol, 84% yield) as semisolid.
References:
PELOTON THERAPEUTICS, INC.;DIXON, Darryl, David;GRINA, Jonas;JOSEY, John, A.;RIZZI, James, P.;SCHLACHTER, Stephen, T.;WALLACE, Eli, M.;WANG, Bin;WEHN, Paul;XU, Rui;YANG, Hanbiao WO2015/35223, 2015, A1 Location in patent:Paragraph 0821

1073-72-9
227 suppliers
$6.00/1g
925-90-6
348 suppliers
$12.00/5ml

67868-84-2
55 suppliers
$18.00/100mg
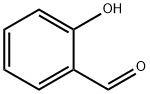
90-02-8
477 suppliers
$9.00/1g