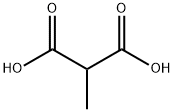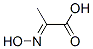
2-(hydroxyimino)-propanoic acid synthesis
- Product Name:2-(hydroxyimino)-propanoic acid
- CAS Number:2211-14-5
- Molecular formula:C3H5NO3
- Molecular Weight:0
127-17-3
566 suppliers
$5.00/10g
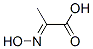
2211-14-5
2 suppliers
inquiry
Yield:2211-14-5 94%
Reaction Conditions:
with hydroxylamine hydrochloride;sodium hydroxide in water at 0 - 20;
Steps:
N-Hydroxyiminopropionic acid (4a)
Oxaloacetic acid (0.4 mL, 5.6 mmol) was added to an aqueous solution containing hydroxylamine hydrochloride (789 mg, 11.34 mmol) and NaOH (681 mg, 17 mmol) at 0°C. The mixture stirred vigorously and was allowed to slowly warm to room temperature. After 18 hrs, the pH was adjusted to 1.0 with concentrated HCl and reaction mixture was extracted with ethyl acetate (3×). The combined organic layers were dried over MgSO4 and concentrated to provide product 4a (550 mg, 94%). 1H NMR (500 MHz, CD3OD) δ 1.99 (s, 3H); 13C NMR (125 MHz, CD3OD) δ 167.4, 149.6, 11.0.
References:
Tibrewal, Nidhi;Elliott, Gregory I. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 1,p. 517 - 519] Location in patent:supporting information; experimental part
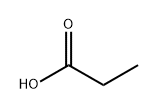
802294-64-0
1 suppliers
inquiry
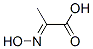
2211-14-5
2 suppliers
inquiry
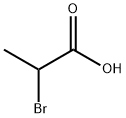
598-72-1
352 suppliers
$16.00/25g
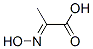
2211-14-5
2 suppliers
inquiry