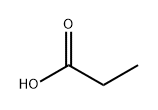
Propionic-2,3-14C1 acid (8CI) synthesis
- Product Name:Propionic-2,3-14C1 acid (8CI)
- CAS Number:802294-64-0
- Molecular formula:C3H6O2
- Molecular Weight:74.08
Yield:802294-64-0 99%
Reaction Conditions:
with bis(acetylacetonato)palladium(II);1,1'-bis(tert-butyl(pyridin-2-yl)phosphanyl)ferrocene;toluene-4-sulfonic acid;acetic acid in water at 120; under 30003 Torr; for 20 h;Inert atmosphere;Autoclave;
Steps:
Variation of the Alkene
General procedure: A 4 ml vial was charged with [Pd(acac)2] (3.07 mg, 0.25 mol %), ligand (1) (20.64 mg, 1.0 mol %), p-toluenesulfonic acid (28.5 mg, 3.75 mol %) and an oven-dried stirrer bar. The vial was then sealed with septa (PTFE-coated styrene-butadiene rubber) and a phenol resin cap. The vial was then connected to the atmosphere with a needle. The vial was evacuated and refilled with argon three times. H2O (0.5 ml), acetic acid (1.5 ml) and alkene (4.0 mmol) were added to the vial with a syringe. The vial was placed in an alloy plate, which was transferred to an autoclave (300 ml) of the 4560 series from Parr Instruments under argon atmosphere. After flushing the autoclave three times with CO, the pressure of CO was increased to 40 bar at room temperature. The reaction was conducted at 120° C. for 20 h. On conclusion of the reaction, the autoclave was cooled down to room temperature and cautiously decompressed. Isooctane (100 μl) was then added as internal standard. Conversion was measured by GC analysis. (0031) The experiment described above was repeated with variation of the alkene. (0032) The results are compiled in the following table 2:
References:
US2019/194110,2019,A1 Location in patent:Paragraph 0029-0032
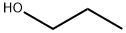
71-23-8
800 suppliers
$12.00/100g

802294-64-0
1 suppliers
inquiry
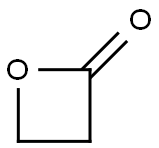
57-57-8
173 suppliers
$24.73/250mgs:

802294-64-0
1 suppliers
inquiry
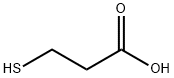
107-96-0
582 suppliers
$6.00/25g

802294-64-0
1 suppliers
inquiry
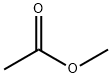
79-20-9
584 suppliers
$14.00/100g
201230-82-2
1 suppliers
inquiry
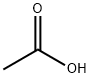
64-19-7
1628 suppliers
$10.00/25ML

802294-64-0
1 suppliers
inquiry
