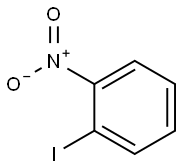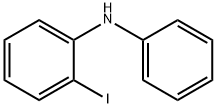
(2-IODO-PHENYL)-PHENYL-AMINE synthesis
- Product Name:(2-IODO-PHENYL)-PHENYL-AMINE
- CAS Number:61613-21-6
- Molecular formula:C12H10IN
- Molecular Weight:295.12
Yield:61613-21-6 78%
Reaction Conditions:
with triethylphosphine in m-xylene at 120; for 3 h;Inert atmosphere;Schlenk technique;Sealed tube;
Steps:
4.1. General procedure
General procedure: A glass culture tube containing a magnetic stir bar was charged with a nitroarene substrate and boronic acid, then fitted with a PTFE-lined silicone septum within a phenolic screw-thread open-top cap after wrapping the reaction tube thread once with Teflon tape. The vessel was evacuated and backfilled with nitrogen on a Schlenk line. Dry m-xylene (2 mL, 0.5 M) was added via syringe, followed by triethylphosphine (0.44 mL, 3 equivalents) and the reaction mixturewas heated 120 °C with stirring. Upon completion, the reaction mixture was cooled to ambient temperature then diluted with 10 mL of distilled water. With the aid of ethyl acetate (ca. 3 x 10 mL), the reaction mixture was transferred to a separatory funnel. After mixing and separating the phases, the organic layer was washed with 10 mL of a 1M NaOH aqueous solution, and 10 mL of brine. Each aqueous phase was back-extracted with one 10 mL portion of ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate, filtered and concentrated by rotary evaporation. The crude residues were purified via column chromatography to yield pure coupling products. Columns were primarily slurry packed with hexanes and mobile phase polarity was increased gradually to the mixture indicated. Note: hexanes = Hex, dichloromethane = DCM, ethyl acetate = EA.
References:
Nykaza, Trevor V.;Yang, Junyu;Radosevich, Alexander T. [Tetrahedron,2019,vol. 75,# 24,p. 3248 - 3252] Location in patent:supporting information
615-43-0
428 suppliers
$5.00/5g
88284-48-4
173 suppliers
$9.00/250mg

61613-21-6
24 suppliers
inquiry