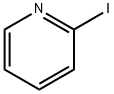
2-Iodopyridine synthesis
- Product Name:2-Iodopyridine
- CAS Number:5029-67-4
- Molecular formula:C5H4IN
- Molecular Weight:205
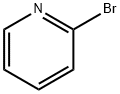
109-04-6

5029-67-4
General procedure for the synthesis of 2-iodopyridine from 2-bromopyridine: the aromatic Finkelstein reaction is used. Since copper(I) iodide is sensitive to moisture and oxygen, the reaction needs to be carried out under argon protection using the standard Schlenk technique. In a two-necked pear-shaped flask equipped with a reflux condenser, (het) aryl bromine feedstock, NaI (using 2 equivalents per bromine exchange) and CuI (using 5 mol% per bromine exchange) were added. N,N'-dimethylethylenediamine (L1) or N,N'-dimethyl-1,2-cyclohexanediamine (L2) (10 mol% per bromine exchange used) and anhydrous 1,4-dioxane (0.5 mL per 1 mmol of NaI used) were subsequently added. The resulting suspension was heated to 110 °C and maintained for 18 hours. After completion of the reaction, it was cooled to room temperature and the mixture was poured into a 25% aqueous NH3 solution. The blue solution was diluted to twice the original volume with H2O and extracted three times with CH2Cl2. For the case of 2,2'-bipyridine, the combined organic phases were additionally washed with aqueous EDTA; otherwise, the combined organic phases were simply washed with brine and dried with MgSO4. The solvent is removed by distillation under reduced pressure to give pure 2-iodopyridine. If necessary, the crude product can be further purified by column chromatography or recrystallization.

109-04-6
521 suppliers
$6.00/25g

5029-67-4
300 suppliers
$6.00/1g
Yield:5029-67-4 92%
Reaction Conditions:
with copper(l) iodide;sodium iodide;N,N`-dimethylethylenediamine in 1,4-dioxane at 110; for 18 h;Inert atmosphere;Schlenk technique;Finkelstein Reaction;
Steps:
Aromatic Finkelstein Reaction; General Procedure
Aromatic Finkelstein Reaction; General ProcedureThe reaction was carried out under argon using standard Schlenktechniques due to the moisture and oxygen sensitivity of the copper(I) iodide. A two-neck pear-shaped flask equipped with a refluxcondenser was charged with the (het)aryl bromide starting material,NaI (2 equiv per bromine to exchange), and CuI (5 mol% per bromineto exchange). N,N′-Dimethylethylenediamine (L1) or N,N′-dimethyl-1,2-cyclohexanediamine (L2) (10 mol% per bromine toexchange) and anhydrous 1,4-dioxane (0.5 mL per 1 mmol NaI)were added. The resulting suspension was heated to 110 °C for 18h. After cooling to r.t., the mixture was poured into aq 25% NH3 solution.The blue solution was diluted to a doubled volume with H2Oand was extracted three times with CH2Cl2. In the case of the 2,2′-bipyridines, the combined organic phases were additionally washedwith aq EDTA solution. Otherwise, the combined organic phaseswere solely washed with brine and dried with MgSO4. The solventwas removed under reduced pressure to give the desired product inpure form. If needed, the crude product can be purified by columnchromatography or recrystallization.
References:
Meyer-Eppler, Georg;Kuechler, Lea;Tenten, Christina;Benkhaeuser, Christian;Brueck, Stefanie;Luetzen, Arne [Synthesis,2014,vol. 46,# 8,art. no. SS-2013-Z0057-OP,p. 1085 - 1090]
109-09-1
0 suppliers
$10.60/1gm:

5029-67-4
300 suppliers
$6.00/1g
13737-05-8
35 suppliers
$155.00/1g

5029-67-4
300 suppliers
$6.00/1g

109-04-6
521 suppliers
$6.00/25g
10034-85-2
342 suppliers
$35.00/25g

5029-67-4
300 suppliers
$6.00/1g
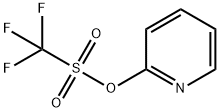
65007-00-3
52 suppliers
$12.00/1g

5029-67-4
300 suppliers
$6.00/1g