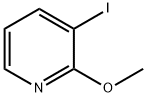
3-Iodo-2-methoxypyridine synthesis
- Product Name:3-Iodo-2-methoxypyridine
- CAS Number:112197-15-6
- Molecular formula:C6H6INO
- Molecular Weight:235.02
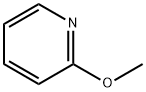
1628-89-3

112197-15-6
The general procedure for the synthesis of 3-iodo-2-methoxypyridine using 2-methoxypyridine as starting material was as follows: firstly, [Li(TMP)Zn(tBu)2] was prepared in 0.4 mmol scale THF solution according to the literature method. To this solution, 2-methoxypyridine (0.042 mL, 0.4 mmol) was added and the reaction mixture was stirred at room temperature for 2 h to obtain a light orange solution. Subsequently, the solution was cooled to 0°C and the reaction was quenched with I2 (508 mg, dissolved in 1 mL THF) and stirring was continued for 1 hour. Upon completion of the reaction, 10% Na2S2O3 solution was added until the solution was bleached and the product was extracted with DCM (3 x 1 mL). The organic phases were combined, dried with MgSO4 and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography using heptane:DCM (20:80→40:60) as eluent to afford 3-iodo-2-methoxypyridine (2a) as a colorless oil (87.1 mg, 92% yield).1H NMR (400 MHz, CDCl3) δ ppm 4.00 (s, 3H), 6.65 (dd, J = 7.61, 4.88 Hz, 1H), 8.03 (dd, J = 7.61, 1.76 Hz, 1H), 8.13 (dd, J = 4.88, 1.76 Hz, 1H). The data obtained are in agreement with literature reports.

1628-89-3
341 suppliers
$17.96/25g

112197-15-6
192 suppliers
$9.00/1g
Yield: 98%
Reaction Conditions:
Stage #1:2-methoxypyridine with 2,2,6,6-tetramethyl-piperidine;n-butyllithium;zinc dichloro(N,N,N′,N′-tetramethylethylenediamine) in tetrahydrofuran;hexane at 20; for 2 h;Inert atmosphere;
Stage #2: with iodine in tetrahydrofuran;hexane at 20;Inert atmosphere;regioselective reaction;Concentration;
Steps:
4.4. General procedure 3
General procedure: To a stirred, cooled (0 C) solution of 2,2,6,6-tetramethylpiperidine (0.25 mL, 1.5 mmol) in THF (2-3 mL) weresuccessively added BuLi (about 1.6 M hexanes solution, 1.5 mmol)and, 5 min later, ZnCl2TMEDA[51] (0.13 g, 0.50 mmol). The mixture was stirred for 15 min at 0 C before introduction of the substrate (0.5 mmol) at 0-10 C. After 2 h at room temperature, a solution of I2 (0.38 g, 1.5 mmol) in THF (4 mL) was added. The mixture was stirred overnight before addition of an aqueous saturated solutionof Na2S2O3 (4 mL) and extraction with AcOEt (320 mL). The combined organic layers were dried over MgSO4, filtered and concentrated under reduced pressure. Purification by chromatography on silica gel (the eluent is given in the product description) led to the compounds described below.
References:
Hedidi, Madani;Bentabed-Ababsa, Ghenia;Derdour, Aïcha;Halauko, Yury S.;Ivashkevich, Oleg A.;Matulis, Vadim E.;Chevallier, Floris;Roisnel, Thierry;Dorcet, Vincent;Mongin, Florence [Tetrahedron,2016,vol. 72,# 17,p. 2196 - 2205]

1628-89-3
341 suppliers
$17.96/25g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

112197-15-6
192 suppliers
$9.00/1g

124-41-4
731 suppliers
$12.00/25g
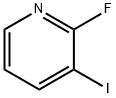
113975-22-7
314 suppliers
$7.00/1g

112197-15-6
192 suppliers
$9.00/1g

67-56-1
790 suppliers
$7.29/5ml-f

113975-22-7
314 suppliers
$7.00/1g

112197-15-6
192 suppliers
$9.00/1g
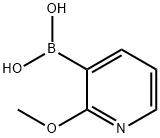
163105-90-6
257 suppliers
$5.00/250mg

112197-15-6
192 suppliers
$9.00/1g