
2-Methoxy-N-methylbenzamide synthesis
- Product Name:2-Methoxy-N-methylbenzamide
- CAS Number:3400-35-9
- Molecular formula:C9H11NO2
- Molecular Weight:165.19

67-56-1
782 suppliers
$9.00/25ml
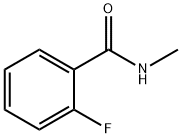
52833-63-3
31 suppliers
$81.06/250mg

3400-35-9
9 suppliers
$131.00/250mg
Yield:3400-35-9 64%
Reaction Conditions:
with potassium hydroxide in dimethyl sulfoxide at 20; for 16 h;
Steps:
3.5 4.2. Typical procedure for the synthesis of 2aa
General procedure: A mixture of 2-fluorobenzamide (1a, 69.5 mg, 0.5 mmol), MeOH (ca. 32.0 mg, 1.0 mmol), KOH (56.0 mg, 1.0 mmol) and DMSO (2.0 mL) in a 25 mL screw-capped thick-walled Pyrex tube was stirred at room temperature for 16 h, and then water (10 mL) was added to the reaction mixture with stirring, and the mixture was extracted with ethyl acetate three times (3 * 10 mL). The combined organic phases were dried over Na2SO4 overnight. The filtered solution was concentrated under reduced pressure, and the crude residue was purified by column chromatography on silica gel with the use of petroleum ether/ethyl acetate/trimethylamine (gradient mixture ratio from 6:1:0.05 to 2:1:0.05 in volume) to afford 2aa as a white solid in 80% yield (60.7 mg).
References:
Su, Ji;Chen, Qian;Lu, Le;Ma, Yuan;Auyoung, George Hong Lok;Hua, Ruimao [Tetrahedron,2018,vol. 74,# 2,p. 303 - 307] Location in patent:supporting information
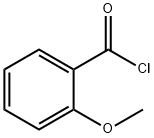
21615-34-9
293 suppliers
$12.00/25g

74-89-5
0 suppliers
$13.44/25ML

3400-35-9
9 suppliers
$131.00/250mg

67-56-1
782 suppliers
$9.00/25ml
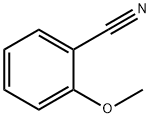
6609-56-9
220 suppliers
$8.00/10g

3400-35-9
9 suppliers
$131.00/250mg

67-56-1
782 suppliers
$9.00/25ml
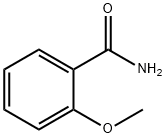
2439-77-2
100 suppliers
$25.00/1g

3400-35-9
9 suppliers
$131.00/250mg
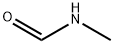
123-39-7
378 suppliers
$5.00/25g
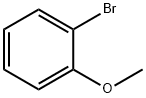
578-57-4
307 suppliers
$6.00/10g

3400-35-9
9 suppliers
$131.00/250mg