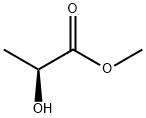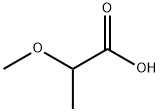
2-methoxypropionic acid synthesis
- Product Name:2-methoxypropionic acid
- CAS Number:4324-37-2
- Molecular formula:C4H8O3
- Molecular Weight:104.1

124-41-4
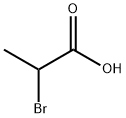
598-72-1

4324-37-2
A methanolic solution of 25% sodium methanolate (16 mL) was slowly added dropwise to a stirring solution of 2-bromopropionic acid (19.6 mmol) in methanol (5 mL) under nitrogen protection. The reaction mixture was heated and reacted at 50 °C overnight while continuously protected by the passage of nitrogen. Upon completion of the reaction, the reaction solution was concentrated under reduced pressure by rotary evaporator. The residue was adjusted to pH 1 with aqueous 1N hydrochloric acid, followed by three extractions with ethyl acetate (70 mL, 25 mL, 10 mL). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated again under reduced pressure to afford 2-methoxypropionic acid as a colorless oil in the yield of 2.04 g (99% yield), which was pure enough to be used directly in the subsequent reaction without further purification. The product was characterized by 1H NMR (CD3OD): δ 3.67 (q, 1H), 3.33 (s, 3H), 1.33 ppm (d, 3H).
Yield: 99%
Reaction Conditions:
in methanol at 50;
Steps:
A.1
Sodium methoxide in methanol (25%, 16 mL) was added to a stirred solution of 2- bromopropionic acid (19.6 mmol) in methanol (5 mL) under nitrogen. The reaction was heated at 50 0C under nitrogen overnight. The reaction was then concentrated under vacuum. The residue was brought to pH 1 by the addition of 1 N aqueous HCl and this solution was then extracted with ethyl acetate three times (70 mL, 25 mL, 10 mL). The combined organic layer was dried (Na2SO4) and then concentrated under vacuum to yield the title compound as a colorless oil 2.04 g (99%) which was of sufficient purity to be used without purification. 1H NMR (CD3OD) δ 3.67 (q, IH), 3.33 (s, 3H), and 1.33 ppm (d, 3H).
References:
BAYER PHARMACEUTICALS CORPORATION WO2006/96338, 2006, A1 Location in patent:Page/Page column 41

67-56-1
787 suppliers
$7.29/5ml-f

124-41-4
723 suppliers
$12.00/25g

598-72-1
346 suppliers
$15.00/25g

4324-37-2
71 suppliers
$9.00/1g

67-56-1
787 suppliers
$7.29/5ml-f
547-64-8
190 suppliers
$15.00/5 mL

4324-37-2
71 suppliers
$9.00/1g

75-07-0
429 suppliers
$14.00/5mL
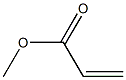
292638-85-8
5 suppliers
inquiry
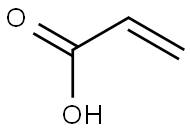
79-10-7
729 suppliers
$20.16/100 mL
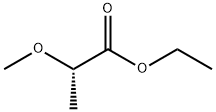
41918-08-5
16 suppliers
$715.00/5g

4324-37-2
71 suppliers
$9.00/1g