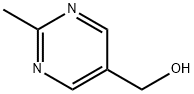
2-Methyl-5-pyrimidinemethanol synthesis
- Product Name:2-Methyl-5-pyrimidinemethanol
- CAS Number:2239-83-0
- Molecular formula:C6H8N2O
- Molecular Weight:124.14
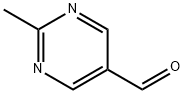
90905-33-2

2239-83-0
2-Methyl-5-pyrimidinecarboxaldehyde (8 g, 66 mmol, compound 44b) was dissolved in methanol (100 mL) at 0°C and sodium borohydride (7.5 g, 197 mmol) was slowly added. The reaction temperature was maintained in the range of 0-15°C and the reaction mixture was stirred for 3 hours. Upon completion of the reaction, the reaction was quenched with saturated ammonium chloride solution (30 mL) and subsequently extracted with ethyl acetate (20 mL x 3). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure. Purification of the residue by silica gel column chromatography afforded (2-methylpyrimidin-5-yl)methanol (4.1 g, 51% yield, compound 44c) as a white solid.
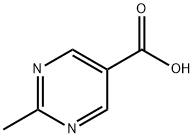
5194-32-1
178 suppliers
$21.89/250mg

2239-83-0
137 suppliers
$21.00/250mg
Yield: 62.6%
Reaction Conditions:
with sodium tetrahydroborate;ethanol at 20; for 3 h;
Steps:
24.1 Step 1 (2-methylpyrimidin-5-yl)methanol
Step 1 (2-methylpyrimidin-5-yl)methanol To a solution of 2-methylpyrimidine-5-carboxylic acid (150 mg, 1.23 mmOl) in ethanol (5 mL) was added sodium borohydride(93 mg, 2.46 mmol). The mixture was stirred at room temperature for 3 h. It was quenched with aqueous HC1 (2 N, 2 mL), extracted with DCM, dried over sodium sulfate, filtered and concentrated give the yellow oil product (2-methylpyrimidin-5-yl)methanol (95 mg, 62.6%). LCMS MH+ 125.
References:
HYDRA BIOSCIENCES, INC.;CHENARD, Bertrand;GALLASCHUN, Randall WO2014/143799, 2014, A2 Location in patent:Page/Page column 387

90905-33-2
135 suppliers
$38.00/500mg

2239-83-0
137 suppliers
$21.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2239-83-0
137 suppliers
$21.00/250mg