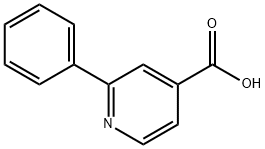
2-PHENYL-ISONICOTINIC ACID synthesis
- Product Name:2-PHENYL-ISONICOTINIC ACID
- CAS Number:55240-51-2
- Molecular formula:C12H9NO2
- Molecular Weight:199.21
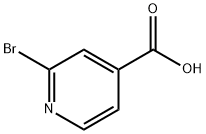
66572-56-3

98-80-6

55240-51-2
Under nitrogen protection, 2-bromo-4-pyridinecarboxylic acid (0.210 g, 1.040 mmol) was dissolved in degassed 1,2-dimethoxyethane (DME, 8 mL). Tetrakis(triphenylphosphine)palladium(0) (0.060 g, 0.052 mmol) was added to the solution and the reaction mixture was stirred for 15 minutes. Subsequently, aqueous potassium carbonate (4.16 mL, 8.32 mmol) and phenylboronic acid (0.171 g, 1.403 mmol) were added. The reaction mixture was refluxed at 95 °C for 18 h, after which it was cooled to room temperature. The reaction mixture was filtered through diatomaceous earth and the pH was adjusted to 3-4 with acid to precipitate a white precipitate, which was filtered and washed with water. The white powdery product 2-phenyl-pyridine-4-carboxylic acid was obtained by recrystallization from 2-methoxyethanol. Yield: 0.106 g, 57% yield. The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 7.44-7.60 (m, 3H), 7.71-7.86 (dd, J = 4.9, 1.5 Hz, 1H), 8.05-8.19 (m, 2H), 8.23-8.35 (t, J = 1.2 Hz, 1H), 8.79-8.93 (dd, J = 5.1, 0.8 Hz, 1H), 13.56-13.97 (s, 1H).UPLC-ESI analysis: retention time 1.37 min, m/z 200.5 [M + H]+ (purity 92%).
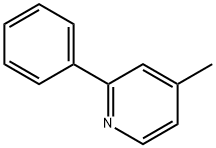
3475-21-6
120 suppliers
$45.00/2.5mg

55240-51-2
104 suppliers
$45.00/10mg
Yield:55240-51-2 84%
Reaction Conditions:
with selenium(IV) oxide in pyridineHeating / reflux;
Steps:
4
2-phenyl-4-methylpyridine (6 g, 35.4 mmol) and SeO2 (24 g, 216 mmol) were refluxed in pyridine (100 ml) overnight under argon. The mixture was then filtered through celite while hot. The Celite filter cake was rinsed with pyridine (3x50 ml) and the resulting filtrate evaporated to dryness. The solid thus obtained was triturated in water (200 ml) and filtered off. The resulting brown solid was suspended in a mixture of water (150 ml) and MeOH (200 ml) and made basic by addition of an aqueous NaOH solution. The mixture was then filtered over Celite to removed some insoluble materials. The filtrate was then acidified with concentrated HCl. MeOH was evaporated and the formed precipitate was filtered, washed with water, then small portions Of Et2O (3x20 ml) and finally dried to afford 5.9 g (84%) of the titled compound as a slightly brown solid.
References:
KONINKLIJKE PHILIPS ELECTRONICS N.V. WO2007/4113, 2007, A2 Location in patent:Page/Page column 24

66572-56-3
353 suppliers
$7.00/5g

98-80-6
745 suppliers
$5.00/5g

55240-51-2
104 suppliers
$45.00/10mg
20662-89-9
84 suppliers
$56.00/1 g
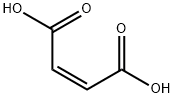
110-16-7
769 suppliers
$10.00/5g

55240-51-2
104 suppliers
$45.00/10mg
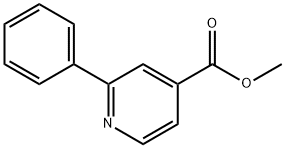
4634-14-4
22 suppliers
inquiry

55240-51-2
104 suppliers
$45.00/10mg

98-80-6
745 suppliers
$5.00/5g

55240-51-2
104 suppliers
$45.00/10mg