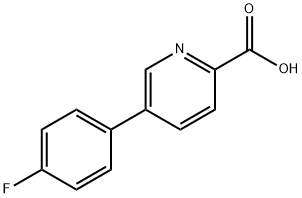
5-(4-Fluorophenyl)pyridine-2-carboxylic acid synthesis
- Product Name:5-(4-Fluorophenyl)pyridine-2-carboxylic acid
- CAS Number:845826-99-5
- Molecular formula:C12H8FNO2
- Molecular Weight:217.2
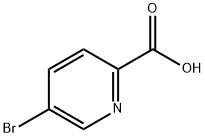
30766-11-1
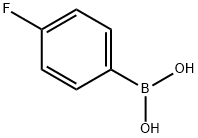
1765-93-1

845826-99-5
Step A: 5-bromopyridine-2-carboxylic acid (57, 1.0 g, 5.0 mmol) was dissolved in a solvent mixture of ethylene glycol dimethyl ether (12 mL) and water (4 mL). Subsequently, 4-fluorophenylboronic acid (17, 1.0 g, 7.5 mmol), anhydrous potassium carbonate (1.0 g, 7.5 mmol) and tetrakis(triphenylphosphine)palladium (289 mg, 0.25 mmol) were added sequentially. The reaction mixture was stirred at 98 °C for 24 h under nitrogen protection. After the completion of the reaction was monitored by thin layer chromatography (TLC), the reaction solution was cooled to room temperature. Water (40 mL) was added to the reaction solution and the pH was adjusted to 2-3 with 6 M hydrochloric acid solution. the precipitate was collected by filtration, the filter cake was redissolved in dichloromethane, and the organic layer was separated and washed with water. The aqueous phase was again adjusted to pH 2-3 with 6 M hydrochloric acid solution and the solid product was collected by filtration. The filter cake was washed with water to neutral and dried to give the crude product. The crude product was treated with 20 mL of saturated sodium bicarbonate solution to give the final target compound 5-(4-fluorophenyl)pyridine-2-carboxylic acid (61,942 mg) in 87.7% yield.

30766-11-1
407 suppliers
$3.00/1g

1765-93-1
552 suppliers
$9.00/1g

845826-99-5
54 suppliers
$140.00/250mg
Yield:845826-99-5 87.7%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in 1,2-dimethoxyethane;water at 98; for 24 h;Inert atmosphere;
Steps:
25.A
Step A:5-bromopyridine-2-carboxylic acid (57, 1.0 g, 5.0 mmol)Dissolved in ethylene glycol dimethyl ether (12 mL) and water (4 mL),Add p-fluorophenylboronic acid (17, 1.0 g, 7.5 mmol)And anhydrous potassium carbonate (1.0 g, 7.5 mmol),Then tetrakis(triphenylphosphine)palladium (289 mg, 0.25 mmol) was added.The resulting mixture was stirred at 98 ° C for 24 hours under a nitrogen atmosphere.TLC analysis indicated that the reaction was over,The reaction solution was cooled to room temperature.Then add water (40 mL),The pH was adjusted to 2-3 with 6M hydrochloric acid.Filtered, the filter cake is dissolved in dichloromethane,The organic layer was washed with 20 mL of saturated sodium bicarbonate solution.Divide the water layer,The aqueous layer was adjusted to pH 2-3 with a 6M hydrochloric acid solution.Filter the solid,The filter cake is washed with water to neutrality.The filter cake was dried to give compound 61 (942 mg).Yield: 87.7%.
References:
CN108623532,2018,A Location in patent:Paragraph 0206; 0207; 0208