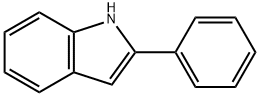
2-Phenylindole synthesis
- Product Name:2-Phenylindole
- CAS Number:948-65-2
- Molecular formula:C14H11N
- Molecular Weight:193.24
Yield:948-65-2 86%
Reaction Conditions:
with acetic acid;zinc(II) chloride in neat (no solvent) at 25 - 180; for 0.416667 h;Fischer Indole Synthesis;Reagent/catalyst;
Steps:
General procedure for the synthesis of 2-phenylindole (4a)
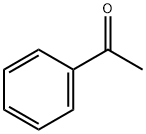
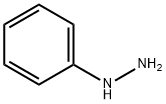
General procedure: Phenylhydrazine 1a (5.1 mmol) and acetophenone 2a (5 mmol) and anhydrous zinc chloride (200 mol %) were mixed together using pestle in mortar and few drops ofacetic acid (0.1 N) was added drop-wise with continuous mixing at room temperature for 10 min. The mixture was transferred into a round-bottomed flask fitted with a reflux condenser attached with CaCl2 guard tube. Then the mixture was heated slowly up to the temperature of 180 °C. The reaction was monitored by TLC using 10% ethyl acetatein n-hexane and the reaction was observed to be completed within 15 min. The mixture was cooled to room temperature and diluted with 5mL dichloromethane and 5mL water. The dichloromethane layer was separated and aqueous layer extracted repeatedly with dichloromethane (3 x 5mL). The combined organic layer was dried over anhydrous Na2SO4, filtered, and solvent was evaporated under reduced pressure. The crude product was purified using flash column chromatography over silica gel using 6% ethyl acetate in hexane as eluent to afford the pure product 2-phenylindole 4a in 86% yield as light yellow solid (Table 1, entry 1). MP 194 °C; 1H-NMR (500 MHz, CDCl3) : 8.33 (brs, 1H), 7.67-7.65 (m, 3H), 7.46-7.39 (m, 3H), 7.35-7.13 (m, 3H), 6.84 (s, 1H); 13CNMR (125 MHz, CDCl3) 137.99, 136.91, 132.45, 129.35, 129.14, 127.83, 125.27, 122.47, 120.79, 120.49, 111.03, 100.08; IR (νmax: 3428.01, 3053.42, 1524.95, 1345.96, 1181.15, 739.99, 685.12 cm-1): HRMS (EI+): m/z calculated for C14H11N [M+H]+: 194.0970, found 194.0964.
References:
Gaikwad, Ruchi;Bobde, Yamini;Ganesh, Routholla;Patel, Tarun;Rathore, Anju;Ghosh, Balaram;Das, Kalpataru;Gayen, Shovanlal [Synthetic Communications,2019,vol. 49,# 17,p. 2258 - 2269]
![1H-Indole, 1-[(4-methylphenyl)sulfonyl]-2-phenyl-](/CAS/20210305/GIF/107734-06-5.gif)
107734-06-5
0 suppliers
inquiry

948-65-2
297 suppliers
$5.00/10g
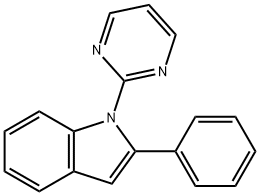
1310708-93-0
0 suppliers
inquiry

948-65-2
297 suppliers
$5.00/10g
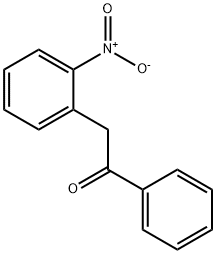
4212-33-3
2 suppliers
inquiry

948-65-2
297 suppliers
$5.00/10g