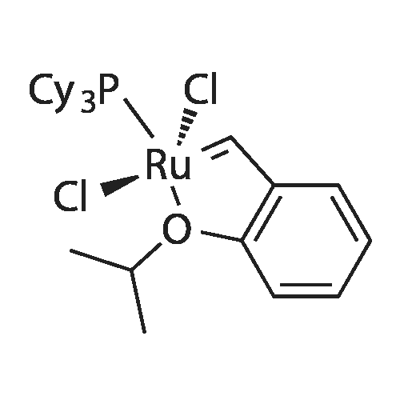
DICHLORO(O-ISOPROPOXYPHENYLMETHYLENE)(TRICYCLOHEXYLPHOSPHINE)RUTHENIUM(II) synthesis
- Product Name:DICHLORO(O-ISOPROPOXYPHENYLMETHYLENE)(TRICYCLOHEXYLPHOSPHINE)RUTHENIUM(II)
- CAS Number:203714-71-0
- Molecular formula:C28H45Cl2OPRu
- Molecular Weight:600.61
172222-30-9
275 suppliers
$9.00/250mg
67191-35-9
45 suppliers
$220.00/500mg

203714-71-0
110 suppliers
$25.50/100mg
Yield:-
Reaction Conditions:
with copper(l) chloride
Steps:
1
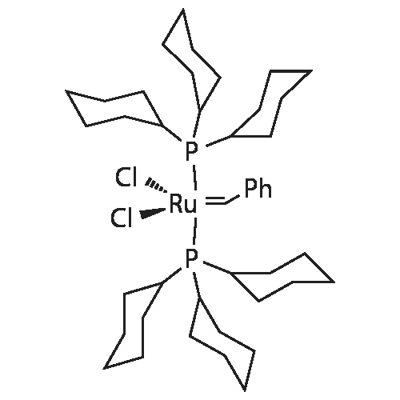
EXAMPLE 1 Metathesis by Ethenolysis of Methyl Oleate Catalyzed by a Type 3 Complex (FIG. 1) in an Ionic Liquid; 1 ml of 3-butyl-1,2-dimethylimidazolium bis-triflylamide with formula [BMMI]+[N(CF3SO2)2]- pre-dried overnight at 80° C., 148 mg of methyl oleate (source: Fluka, with a purity higher than 98%) and 15 mg of the complex with formula Cl2Ru(CH-o-O-iPrC6H4)PCy3 (synthesized by reacting the 1st generation Grubbs complex with formula Cl2Ru(CHC6H5)(PCy3)2 with 1-isopropoxy-2-vinylbenzene in the presence of CuCl), this corresponding to 5% molar of catalyst with respect to methyl oleate, were introduced, in an inert atmosphere of argon, into an autoclave reactor provided with an agitation system and a pressure sensor. The autoclave was then placed under vacuum and pressurized to obtain a pressure of 10 bars (1 MPa) of ethylene (origin: Alphagas, quality N25). The temperature was kept constant at 20° C. The medium was stirred at ambient temperature for 2 hours, then the excess ethylene was slowly purged by returning to atmosphere pressure at a temperature not exceeding 20° C. and the autoclave was again placed under an atmosphere of argon. The products were separated from the ionic liquid by adding 2 to 3 ml of heptane distilled over CaH2 and degassed. An aliquot (100 μl) of the extracted solution was passed through a short silica column (2 cm) eluted with diethyl ether. It was analyzed by gas phase chromatography (ZB-1 column, 100% dimethylpolysiloxane, 30 metres, helium vector gas 2 ml/min, temperature programming: 60° C. then 5° C./min to 220° C.) coupled to a mass spectrometer. The methyl oleate conversion was 95%. It was calculated using decane as an internal reference. The reaction products were composed of 1-decene (fraction A) and methyl decenoate (fraction B). The presence of 1-decene isomers was not detected. Homo-metathesis products were present in trace amounts and could not be quantified.
References:
Olivier-Bourbigou, Helene;Vallee, Christophe;Hillion, Gerard US2007/179307, 2007, A1 Location in patent:Page/Page column 3-4