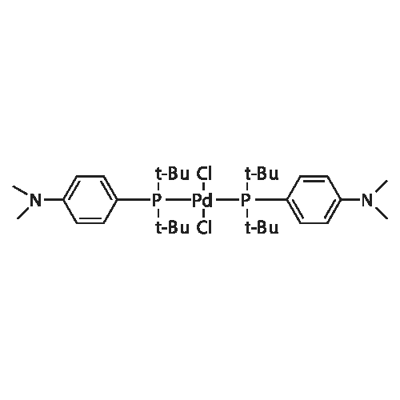
Bis(di-tert-butyl(4-dimethylaminophenyl)phosphine)dichloropalladium(II) synthesis
- Product Name:Bis(di-tert-butyl(4-dimethylaminophenyl)phosphine)dichloropalladium(II)
- CAS Number:887919-35-9
- Molecular formula:C32H58Cl2N2P2Pd
- Molecular Weight:710.1
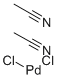
14592-56-4
337 suppliers
$22.00/100mg
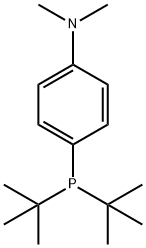
932710-63-9
226 suppliers
$11.00/250mg

887919-35-9
294 suppliers
$20.00/250mg
Yield:887919-35-9 1445 g
Reaction Conditions:
in tetrahydrofuran at 20; for 9.5 h;Inert atmosphere;
Steps:
1
Take a dry 10L three-neck reaction flask, dry nitrogen is fully replaced and under nitrogen flow protection,Add 5.5 L of anhydrous tetrahydrofuran, stir and add 540 g of bis(acetonitrile)palladium dichloride.The obtained ligand di-tert-butyl-4-dimethylaminophenylphosphine was further reacted for 30 minutes, and a yellow solid was precipitated. After the reaction was continued for 9 hours at room temperature,After filtration, the filter cake was dipped in anhydrous tetrahydrofuran, drained and dried in a vacuum oven at 60 ° C.Obtaining a yellow crystalline powdery target product, namely dichlorodi-tert-butyl-(4-dimethylaminophenyl)phosphine palladium, yielding 1440 g to 1445 g,Elemental analysis showed that the product content exceeded 98.0%, the palladium content exceeded 15.0%, and the palladium calculated yield was 97.7% to 98.1%.
References:
CN108659054,2018,A Location in patent:Paragraph 0057; 0061
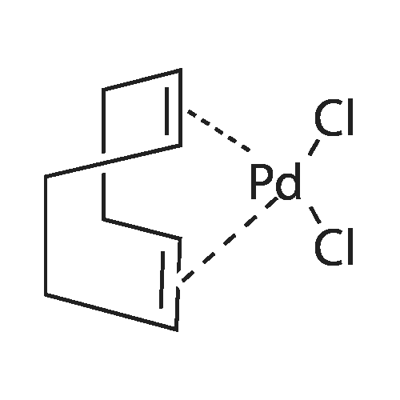
12107-56-1
260 suppliers
$30.80/250mg

932710-63-9
226 suppliers
$11.00/250mg

887919-35-9
294 suppliers
$20.00/250mg
![Magnesium, chloro[4-(dimethylamino)phenyl]-](/CAS/20211123/GIF/108949-55-9.gif)
108949-55-9
0 suppliers
inquiry

887919-35-9
294 suppliers
$20.00/250mg
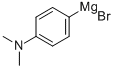
7353-91-5
74 suppliers
$226.00/50mL

887919-35-9
294 suppliers
$20.00/250mg
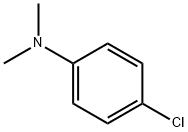
698-69-1
76 suppliers
$17.00/250mg

887919-35-9
294 suppliers
$20.00/250mg