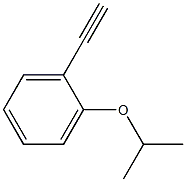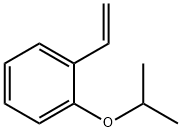
Benzene, 1-ethenyl-2-(1-Methylethoxy)- synthesis
- Product Name:Benzene, 1-ethenyl-2-(1-Methylethoxy)-
- CAS Number:67191-35-9
- Molecular formula:C11H14O
- Molecular Weight:162.23
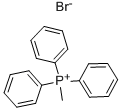
1779-49-3
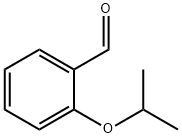
22921-58-0

67191-35-9
1-Isopropoxy-2-vinylbenzene was synthesized as follows: first, 15.65 g of methyltriphenylphosphonium bromide was suspended in 100 mL of dry tetrahydrofuran (THF), and 19.0 mL of n-butyllithium (n-BuLi, 2.5 M n-hexane solution) was slowly added to this suspension at room temperature. The reaction mixture was then transformed into an orange colored solution with continuous stirring for 4 hours. Subsequently, 6.00 g (36.5 mmol) of 2-isopropoxybenzaldehyde dissolved in 25 mL of anhydrous THF was slowly added dropwise under stirring. Upon completion of the dropwise addition, the formation of a white precipitate was observed in the reaction system. After continued stirring of the reaction mixture for 1 h, vacuum concentration was carried out to give a viscous yellow oil. The crude product was purified by a short silica gel column (eluent: heptane) followed by vacuum distillation to give 3.66 g of 1-isopropoxy-2-vinylbenzene as a colorless liquid. The structure of the product was confirmed by 1H-NMR (300 MHz, CDCl3) and GC-MS analysis: 1H-NMR (300 MHz, CDCl3) δ 1.27 (d, J = 6.1 Hz, 6H), 4.47 (m, 1H), 5.15 (dd, J1 = 11.2 Hz, J2 = 1.6 Hz, 1H), 5.65 (dd, J1 = 17.8 Hz, J2 = 1.6 Hz, 1H), 6.78-6.88 (m, 2H), 6.92-7.05 (m, 1H), 7.08-7.16 (m, 1H), 7.40 (dd, J1 = 7.6 Hz, J2 = 1.7 Hz, 1H); GC-MS showed purity of >99.0%, MS (EI): 162.

1779-49-3
626 suppliers
$5.00/10g

22921-58-0
79 suppliers
$42.00/500mg

67191-35-9
45 suppliers
$220.00/500mg
Yield:67191-35-9 3.66 g
Reaction Conditions:
Stage #1:Methyltriphenylphosphonium bromide with n-butyllithium in tetrahydrofuran;hexane at 20; for 4 h;
Stage #2:2-isopropoxybenzaldehyde in tetrahydrofuran;hexane for 1 h;
Steps:
Synthesis of 1-isopropoxy-2-vinylbenzene.
Synthesis of 1-isopropoxy-2-vinylbenzene. A suspension of 15.65 g of methyl triphenylphosphonium bromide in 100 mL of dry THE was treated at room temperature with 19.0 mL of n-BuLi (2.5 M solution in n-hexane). The resulting orange solution was stirred for 4 hours. Then, a solution of 6.00 g (36.5mmol) of 2-isopropoxybenzaldehyde in 25 mL of dry THE was added dropwise. Upon addition, a white precipitate formed. The suspension was stirred for 1 hour and concentrated in vacuo to give a viscous yellow oil that was purified by passing through a short column of Si02 (heptane) then vacuum distillation to yield 3.66 g of 1-isopropoxy-2-vinylbenzene as a colorless liquid. 1H-NMR (300 MHz, CDCI3): 51.27 (d, J=6.1 Hz, 6H), 4.47 (m, 1H), 5.15 (dd, Ji=11.2 Hz, J2=1.6 Hz, 1H), 5.65 (dd, Ji=17.8Hz, J2=1.6 Hz, IH), 6.78-6.88 (m, 2H), 6.92-7.05 (m, 1H), 7.08-7.16 (m, IH), 7.40 (dd, J1=7.6 Hz, J2=1.7 Hz, 1H). GC-MS: >99.0% MS (El): 162.
References:
XIMO AG;TOTH, Florian;FRÁTER, Georg;ONDI, Levente WO2015/155593, 2015, A1 Location in patent:Page/Page column 50
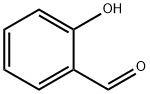
90-02-8
477 suppliers
$9.00/1g

67191-35-9
45 suppliers
$220.00/500mg
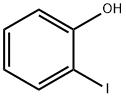
533-58-4
383 suppliers
$6.00/5g
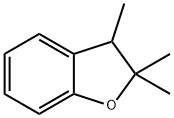
70201-81-9
0 suppliers
inquiry

67191-35-9
45 suppliers
$220.00/500mg
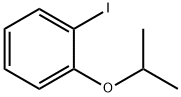
857335-90-1
11 suppliers
inquiry

70201-81-9
0 suppliers
inquiry

67191-35-9
45 suppliers
$220.00/500mg