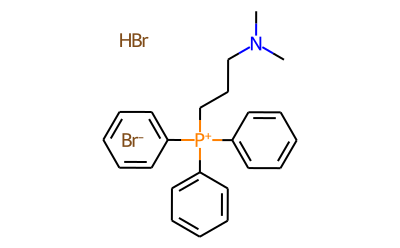
[3-(Dimethylamino)propyl]triphenylphosphonium bromide hydrobromide synthesis
- Product Name:[3-(Dimethylamino)propyl]triphenylphosphonium bromide hydrobromide
- CAS Number:27710-82-3
- Molecular formula:C23H28Br2NP
- Molecular Weight:509.27
3607-17-8

124-40-3
![[3-(Dimethylamino)propyl]triphenylphosphonium bromide hydrobromide](/CAS/GIF/27710-82-3.gif)
27710-82-3
To a suspension of (3-bromopropyl)triphenylphosphonium bromide (1.0 g, 2.1 mmol) in ethanol (5 mL) was slowly added 40% aqueous dimethylamine (3 mL) at room temperature. The reaction mixture was transferred to a sealed microwave reaction tube and heated at 100 °C with stirring for 30 min. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure to give the crude product. The crude product was purified by recrystallization in acetonitrile to afford [3-(dimethylamino)propyl]triphenylphosphonium bromide hydrobromide (0.90 g, 82% yield) as a white solid, which was used directly in the subsequent reaction.ESI MS m/z 348.3 (corresponding to Ph3PCH2CH2CH2CH2NMe2+ ions).

3607-17-8
216 suppliers
$8.00/10g

124-40-3
545 suppliers
$18.00/100ml
![[3-(Dimethylamino)propyl]triphenylphosphonium bromide hydrobromide](/CAS/GIF/27710-82-3.gif)
27710-82-3
202 suppliers
inquiry
Yield:27710-82-3 92.4%
Reaction Conditions:
in ethanol;Reflux;Temperature;
Steps:
17-32; 2 Comparative Example 2. Synthesis of [3-(Dimethylamine)propyl]triphenylphosphonium bromide hydrobromide
General procedure: Add 720 g of absolute ethanol to the reaction flask, add about 300 g of 123B1-10 obtained in Comparative Example 1, add 450 g of 40% dimethylamine aqueous solution, and heat and reflux for 4 to 5 hours; after the reaction is completed, concentrate the dry solvent under reduced pressure to obtain Add 400g of anhydrous ethanol to the residue, stir and heat to reflux to disperse the agglomerated solids; cool and crystallize; filter, collect the solids and dry to obtain about 273g of the product 123B2-10. Single-pass yield: 82.9%. HPLC: 99.92%; where: 123B2-10: triphenylphosphine: 123B1-X2=99.97:0.00:0.03 (excluding other impurities).The mother liquor is collected and recovered to obtain about 31 g of the product 123B2-10. Recovery rate: 9.4%. HPLC: 99.63%; where: 123B2-10: triphenylphosphine: 123B1-X2=99.92:0.00:0.08 (excluding other impurities).A total of about 304g of 123B2-10 is obtained. The total yield of ammonolysis: 92.4%.
References:
CN111548369,2020,A Location in patent:Paragraph 0048-0072; 0076-0083