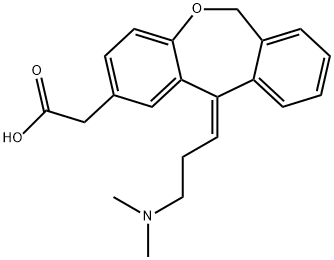
Olopatadine synthesis
- Product Name:Olopatadine
- CAS Number:113806-05-6
- Molecular formula:C21H23NO3
- Molecular Weight:337.41
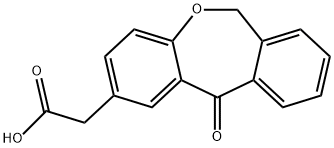
55453-87-7
348 suppliers
$5.00/25mg
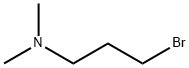
53929-74-1
50 suppliers
inquiry

113806-05-6
139 suppliers
$89.00/25mg
Yield:113806-05-6 81.2%
Reaction Conditions:
Stage #1:N,N-dimethyl-3-bromopropylamine with zinc dibromide in tetrahydrofuran at 10 - 20; for 0.166667 h;Inert atmosphere;
Stage #2: with naphthalene;lithium in tetrahydrofuran at 65 - 70;Inert atmosphere;
Stage #3:isoxepac in tetrahydrofuran at 0 - 25; for 16.5 h;Reagent/catalyst;Temperature;
Steps:
2 Example 2
Lithium naphthalene reagent preparation Lithium metal (0.4 g, 60 mmol) was added to anhydrous naphthalene (2.6 g, 20 mmol) under nitrogen protection In the temperature control 20-25 ° C stirring 2h lithium naphthalene reagent.Preparation of highly active organozinc reagent Under nitrogen protection, 3-bromo-N,N-dimethylpropanamine (5.0 g, 30 mmol) was added to the reaction flask, anhydrous tetrahydrofuran (32 ml), and the mixture was stirred and cooled to 10-20 °C. Zinc bromide (13.5 g, 60 mmol) was added in batches, stirred for 10 minutes, and the above-mentioned naphthalene lithium reagent was added dropwise. After the reaction temperature was stabilized, the mixture was heated to reflux (65-70° C.), and the reaction was stirred for 5-6 h. The resulting reaction solution is a highly active organozinc reagent.Preparation of Crude Olopatadine Isoxepac(4.0g, 15mmol) was dissolved in tetrahydrofuran (12ml), dissolved and clarified, and the temperature was controlled at 0-10°C.In the above high activity organozinc reagent, after about 0.5 h, the reaction mixture was heated to 20-25° C. and stirred for 16 h. The end point of the reaction was followed by HPLC, and the purity was 94.0%. The reaction solution was cooled, and 25 ml of water was added for extraction. The pH of the aqueous phase was adjusted to pH 4.0 to 4.4. The aqueous phase was washed with n-hexane, methyl tert-butyl ether, and ethyl acetate, respectively, and n-butanol was extracted with 20 ml×4 of water to decolorize. Evaporated under reduced pressure to give 4.4 g of oil, with a purity of 96.0%.Refined OlrotidineTo the above-obtained oily substance, n-butanol (20 ml) was added, heated to 75-80°C, and stirred for 15 minutes.But to 5-10 ° C, stirring crystallized 1h, filtered and dried to give 3.6g olopatadine, yield 81.2%, purity 99.2%.
References:
Beijing Huaxi Joint Science And Technology Co., Ltd.;Tian Liwen;Xie Xiaodong;Bi Hua CN103664861, 2018, B Location in patent:Paragraph 0027; 0036-0038; 0042-0051; 0054-0056
113806-01-2
11 suppliers
inquiry

113806-05-6
139 suppliers
$89.00/25mg

55453-87-7
348 suppliers
$5.00/25mg
![[3-(Dimethylamino)propyl]triphenylphosphonium bromide hydrobromide](/CAS/GIF/27710-82-3.gif)
27710-82-3
202 suppliers
inquiry

113806-05-6
139 suppliers
$89.00/25mg
![Dibenz[b,e]oxepin-2-acetamide, 11-[3-(dimethylamino)propylidene]-6,11-dihydro-, (11Z)-, 4-methylbenzenesulfonate (1:1)](/CAS/20211123/GIF/1123338-92-0.gif)
1123338-92-0
1 suppliers
inquiry

113806-05-6
139 suppliers
$89.00/25mg
![(Z)-11-(3-dimethylaminopropylidene)-6,11-dihydro-dibenz-[b,e]oxepine-2-acetic acid hydrobromide](/CAS/20200331/GIF/951006-73-8.gif)
951006-73-8
1 suppliers
inquiry

113806-05-6
139 suppliers
$89.00/25mg