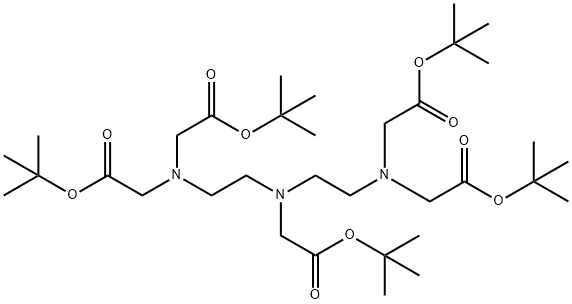
tetra-tert-butyl 2,2',2'',2'''-((((2-(tert-butoxy)-2-oxoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanetriyl))tetraacetate synthesis
- Product Name:tetra-tert-butyl 2,2',2'',2'''-((((2-(tert-butoxy)-2-oxoethyl)azanediyl)bis(ethane-2,1-diyl))bis(azanetriyl))tetraacetate
- CAS Number:280563-33-9
- Molecular formula:C34H63N3O10
- Molecular Weight:673.88
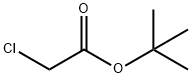
107-59-5
288 suppliers
$27.00/25g
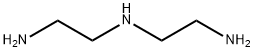
111-40-0
526 suppliers
$13.44/25ML

280563-33-9
9 suppliers
$766.00/1g
Yield:280563-33-9 61%
Reaction Conditions:
with potassium carbonate in acetonitrile; for 24 h;
Steps:
3
EXAMPLE 3; A Synthesis of a penta-tert-butyl DTPA; Please refer to FIG. 4, which is a view of the structure of a penta-tert-butyl DTPA according to the present invention. A diethylenetriamine (2 g, 9.69 millimole), a potassium carbonate (6.7 g) and a 70 ml of acetonitrile are placed in a flask as being stirred. And, a tert-butyl chloroacetate (8.32 ml, 58.16 millimole) dissolved in a 10 ml of acetonitrile is slowly added into the flask to process reactions for 24 hours. Then, the solvent of the solution is removed and the solution is filtrated. The ethyl ether and the hexane are mixed under a mixture rate of 1:10 in a chromatographic column to be separated and purified to obtain a 4.0 g of penta-tert-butyl DTPA 4, whose yield rate is 61%. 1H NMR (CDCl3/δ ppm) δ 1.36 (45H 5×-O-C-(CH3)3) δ 2.71 (S 8H 2×-N-CH2-CH2-N) δ 3.273.45 (10H 5×-N-CH2-CO) 13C NMR (CDCl3/δ ppm) δ 28.06 δ 55.97 δ 80.72 δ 52.09 δ 76.61 δ 70.55 δ 52.68 δ 77.03 δ 55.67 δ 77.46 EIMS (m/e %) (MH+)=676 EA (C, H, N %) Calculated: C: 60.60% H: 9.42% N: 8.31% Found: C: 61.72% H: 9.51% N: 8.09%
References:
US2006/264669,2006,A1 Location in patent:Page/Page column 3
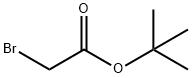
5292-43-3
445 suppliers
$10.00/5g

111-40-0
526 suppliers
$13.44/25ML

280563-33-9
9 suppliers
$766.00/1g