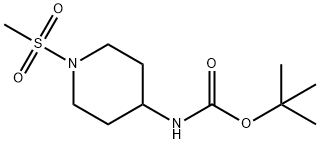
tert-Butyl (1-(Methylsulfonyl)piperidin-4-yl)carbaMate synthesis
- Product Name:tert-Butyl (1-(Methylsulfonyl)piperidin-4-yl)carbaMate
- CAS Number:287953-38-2
- Molecular formula:C11H22N2O4S
- Molecular Weight:278.37

124-63-0
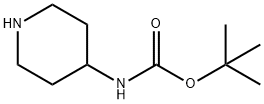
73874-95-0

287953-38-2
General procedure for the synthesis of 1-methanesulfonyl-4-tert-butoxycarbonylaminopiperidine from methanesulfonyl chloride and 4-tert-butoxycarbonylaminopiperidine: 5.00 g (25.0 mmol) of Boc-4-aminopiperidine was dissolved in 19.8 mL of pyridine and cooled in an ice bath. 2.13 mL (27.5 mmol) of methanesulfonyl chloride was added slowly and dropwise. The reaction mixture was stirred at room temperature for 16 hours. After completion of the reaction, the reaction mixture was diluted with water and then extracted with dichloromethane (DCM). The organic layer was washed with water, dried over anhydrous magnesium sulfate (MgSO4) and filtered. The solvent was removed by concentration under reduced pressure to give 6.30 g of tert-butyl 1-methanesulfonyl-piperidin-4-yl-carbamate. The yield was 91%; ESI-MS: 279 [M + H]+.

124-63-0
0 suppliers
$10.00/5g

73874-95-0
440 suppliers
$10.00/250mg

287953-38-2
44 suppliers
$36.00/250mg
Yield: 91%
Reaction Conditions:
with pyridine at 20; for 16 h;Cooling with ice;
Steps:
1 Step 1 : (1 -Methanesulfonyl-piperidin-4-yl)-carbamic acid tert-butyl ester
5.00 g (25.0 mmol) of BOC-4-aminopiperidine are dissolved in pyridine (19.8 mL) and cooled in an ice bath. 2.13 mL (27.5 mmol) of methanesulfonyl chloride are added slowly. The reaction is stirred at RT for 16 h. After diluting with water, the reaction is extracted with DCM. Organic layers are washed with water, dried with MgS04 and filtered. The solvent is removed under reduced pressure to afford 6.30 g of (1 -Methanesulfonyl-piperidin-4-yl)-carbamic acid tert-butyl ester. Yield: 91 %; ESI-MS: 279 [M+H]+
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;RIETHER, Doris;BINDER, Florian;DOODS, Henri;MUELLER, Stephan, Georg;NICHOLSON, Janet, Rachel;SAUER, Achim WO2014/184327, 2014, A1 Location in patent:Page/Page column 27
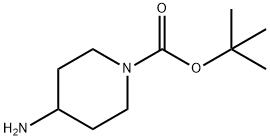
87120-72-7
0 suppliers
$6.00/1g

124-63-0
0 suppliers
$10.00/5g

287953-38-2
44 suppliers
$36.00/250mg