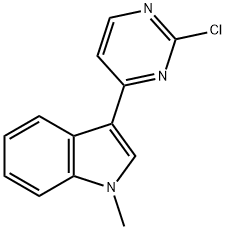
3-(2-chloropyriMidin-4-yl)-1-Methylindole synthesis
- Product Name:3-(2-chloropyriMidin-4-yl)-1-Methylindole
- CAS Number:1032452-86-0
- Molecular formula:C13H10ClN3
- Molecular Weight:243.69
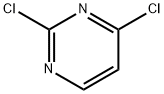
3934-20-1
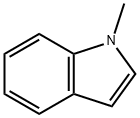
603-76-9

1032452-86-0
The general procedure for the synthesis of 3-(2-chloropyrimidin-4-yl)-1-methylindole from 2,4-dichloropyrimidine and N-methylindole is as follows: Intermediate 7: Synthesis of 3-(2-chloropyrimidin-4-yl)-1-methylindole 40g of 2,4-dichloropyrimidine and 200mL of 1,2-dimethoxyethane (DME) were added to a 1L three-neck flask and stirred until complete dissolution. After cooling the reaction system to 10-15°C, 45 g of anhydrous ferric chloride (FeCl3) was added rapidly and in batches, controlling the reaction temperature to not exceed 35°C. Stirring of the reaction mixture was continued for 15 minutes after each portion of FeCl3 was added. Subsequently, 52.8 g of N-methylindole was slowly added dropwise to the reaction system. After the dropwise addition, the reaction mixture was slowly heated to 50°C and stirred at this temperature overnight. The progress of the reaction was monitored by thin layer chromatography (TLC) until the reaction was completed. Upon completion of the reaction, the mixture was cooled to 5-10°C and about 300 mL of a mixture of methanol and water (1:2 by volume) was slowly added dropwise, at which time a large amount of viscous solid precipitated. The reaction mixture was filtered, the solid product was collected and the filter cake was washed twice with methanol. Finally, the resulting solid was dried under reduced pressure at 50°C to give the target product in 85% yield.
945016-63-7
81 suppliers
$44.00/100mg

74-88-4
357 suppliers
$15.00/10g

1032452-86-0
306 suppliers
$9.00/1g
Yield:1032452-86-0 96%
Reaction Conditions:
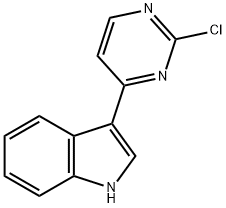
Stage #1: 3-(2-chloro-pyrimidin-4-yl)-1H-indolewith sodium hydride in tetrahydrofuran;mineral oil at 0; for 0.5 h;
Stage #2: methyl iodide in tetrahydrofuran;mineral oil at 0; for 3 h;
Steps:
130 Intermediate 130: 3-(2-Chloropyrimidin-4-yl)-1-methylindole
NaH (1.707 g, 42.68 mmol, 40% dispersion in mineral oil) was added in small portions to a cooled (0°C) mixture of 3-(2-chloropyrimidin-4-yl)-1H-indole (Intermediate 131, 8.168 g, 35.57 mmol) in THF (250 mL). The resulting mixture was stirred at 0°C for 0.5h and then CH3I (2.67 mL, 42.68 mmol) was added and the mixture stirred at 0°C for a further 3h. The reaction was quenched by the addition of sat. NaHCO3 (25 mL). The mixture was then diluted with EtOAc (100 mL), and the resulting solution was washed with sat. NaHCO3 (50 mL), water (50 mL) and sat. brine (50 mL). The organic solution was then concentrated in vacuo. Purification by FCC, eluting with 0-20% CH3OH in CH2Cl2 gave the title compound (8.35 g, 96%) as a pale yellow solid; 1H NMR: 3.90 (3H, s), 7.30 (2H, pd), 7.54-7.60 (1H, m), 7.82 (1H, d), 8.38-8.44 (1H, m), 8.49 (1H, s), 8.53 (1H, d); m/z: ES+ MH+ 244.
References:
WO2013/14448,2013,A1 Location in patent:Page/Page column 138

3934-20-1
725 suppliers
$9.00/1g

603-76-9
435 suppliers
$18.00/5g

1032452-86-0
306 suppliers
$9.00/1g

945016-63-7
81 suppliers
$44.00/100mg

50-00-0
896 suppliers
$10.00/25g

1032452-86-0
306 suppliers
$9.00/1g
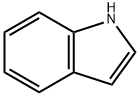
120-72-9
658 suppliers
$6.00/25g

3934-20-1
725 suppliers
$9.00/1g

75-16-1
274 suppliers
$12.00/10ml

1032452-86-0
306 suppliers
$9.00/1g

120-72-9
658 suppliers
$6.00/25g

1032452-86-0
306 suppliers
$9.00/1g