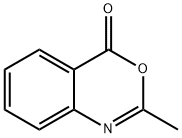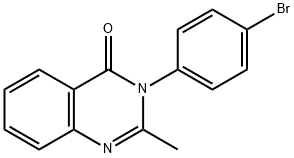
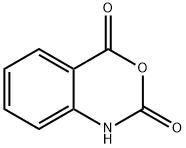
3-(4-BROMOPHENYL)-2-METHYLQUINAZOLIN-4(3H)-ONE synthesis
- Product Name:3-(4-BROMOPHENYL)-2-METHYLQUINAZOLIN-4(3H)-ONE
- CAS Number:1788-95-0
- Molecular formula:C15H11BrN2O
- Molecular Weight:315.16
Yield:1788-95-0 95.13%
Reaction Conditions:
Stage #1: 2-methyl-4-oxo-3,1-benzoxazine in ethanol at 20; for 0.0833333 h;
Stage #2: 4-bromo-aniline in ethanol at 100; for 6 h;
Steps:
General procedure for synthesis of 2-methyl-3-substituted quinazolin-4(3H)-ones, 8a-q.
General procedure: 2-Methyl-4H-3,1-benzoxazin-4-one, 7 (3.00g, 18.60 mmol) was dissolved in 10 ml of ethanol ina 250 ml round bottom ask and the reaction mixturewas allowed to stir for 5 mins. at room temperature forcomplete dissolution, followed by the addition of thecorresponding amino containing compounds (18.60mmol). The mixture was then heated under reux forthe required number of periods as determined by the TLC monitoring. The reaction mixture was allowedto cool, fltered by suction and air-dried to affordmoderate to excellent yields of various 2-methyl-3-substituted quinazolin-4(3H)-one derivatives, 8a-q.
References:
Ajani, Olayinka O.;Audu, Oluwatosin Y.;Germann, Markus W.;Bello, Babatunde L. [Oriental Journal of Chemistry,2017,vol. 33,# 2,p. 562 - 574]
615-43-0
426 suppliers
$5.00/5g

108-24-7
0 suppliers
$14.00/250ML
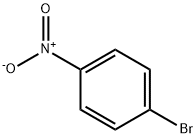
586-78-7
19 suppliers
$14.00/5g

1788-95-0
13 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML
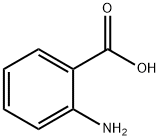
118-92-3
0 suppliers
$23.00/250g

106-40-1
483 suppliers
$12.00/5g

1788-95-0
13 suppliers
inquiry
118-48-9
441 suppliers
$5.00/5G
78-39-7
374 suppliers
$9.00/1g

106-40-1
483 suppliers
$12.00/5g

1788-95-0
13 suppliers
inquiry