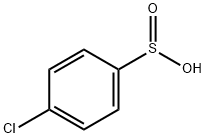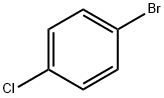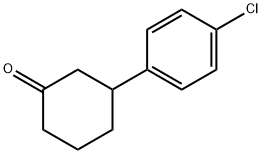
3-(4-chlorophenyl)cyclohexanone synthesis
- Product Name:3-(4-chlorophenyl)cyclohexanone
- CAS Number:136333-71-6
- Molecular formula:C12H13ClO
- Molecular Weight:208.68
Yield:136333-71-6 99%
Reaction Conditions:
with lithium hydroxide;(η6-1,4-dihydroxybenzene)(η2,η2-cyclooctadiene-1,5)rhodium tetrafluoroborate in 1,2-dimethoxyethane;water at 50; for 1 - 3 h;
Steps:
This study demonstrates that base is required for the reaction and 2.0 mol % provides optimal yield of the desired conjugate addition product. During further studies to optimize the reaction conditions, a series of additives and bases were examined. Catalytic amounts of carbonate bases, Na2CO3 (2.0 mol %) or Cs2CO3 (2.0 mol %), are effective at producing high yielding conjugate additions with boronic acid 4a (Table 3, entries 6 & 8), while stoichiometric amounts (120 mol %) of carbonate bases (entries 7 & 9) attenuated reactivity. Pyridine, either catalytic or quantitative, arrests all reactivity and consistent with this observation is the lack of product with pyridine boronic acids. Additional hydroquinone shows no detectable effect upon reaction outcome while lithium salts, such as LiCl or LiBF4, either diminish the amount of product or completely arrest the reaction. The addition reaction can be run in the absence of organic solvent, however, stoichiometric base (120 mol %) is required for efficient reaction (entry 10 versus 11). This result is presumably due to the solubilization of the boronic acid into the aqueous phase by formation of the corresponding-ate complex. Preferred reaction conditions, outlined as a general procedure in the experimental section, are highly effective and facile for a range of boronic acid substrates. Using 2-cyclohexen-1-one as our conjugate acceptor, a number of different aryl boronic acids were studied with our optimized reaction conditions (Table 4). Ketone products 6a-g (entries 1-7) are afforded in high yields, with low catalyst loading (0.5 mol %) and low boronic acid equivalency (1.2 eq). Electron deficient boronic acids (entries 5-9) are afforded in excellent yields (94-99%) without any procedural modification from the earlier analogues. Improved yields (92-93%) of meta-nitro analogue 6i were achieved either by increasing the catalyst loading (2.0 mol %, entry 12) or increasing equivalencies of boronic acid (1.5 equiv, entry 13). Tri-fluoro analogues 6j and 6k (entry 14,15) were afforded in good to moderate yields (70% and 30% respectively). This is believed to be the first report of conjugate addition of a tri-halogenated aryl boronic acid. Efforts are underway to further optimize the additions of tri-fluorophenyl boronic acids 4j and 4k. Both 2,4-bis(trifluoromethyl)phenyl boronic acid and ortho-nitro phenyl boronic acid failed to produce the desired addition products under our standard conditions. The 4-, 5- or 6-indoloboronic acids (Table 5) undergo conjugate addition while N-Boc-2-indoloboronic acid (entry 1) does not afford any product. The additions of 4-indoloboronic acid (entry 2, Table 4) and o-tolyl boronic acid (entry 3, Table 4) show that ortho substitution can be tolerated, despite the attenuated reactivity observed for o-substituted boronic acids and documented difficulties of reactions with N-Boc protected pyrrole-2-boronic acids. See Lautens, M.; Mancuso, J.; Grover, H. Synthesis 2004, 12, 2006-2014.
References:
US2007/123715,2007,A1 Location in patent:Page/Page column 13; 14
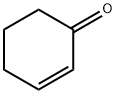
930-68-7
262 suppliers
$9.00/1g
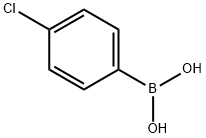
1679-18-1
619 suppliers
$10.00/1g

91398-51-5
4 suppliers
inquiry

136333-71-6
12 suppliers
inquiry

1679-18-1
619 suppliers
$10.00/1g
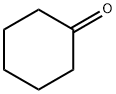
108-94-1
581 suppliers
$12.00/50g

136333-71-6
12 suppliers
inquiry