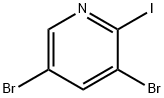
3,5-DibroMo-4-iodopyridine synthesis
- Product Name:3,5-DibroMo-4-iodopyridine
- CAS Number:1214383-75-1
- Molecular formula:C5H2Br2IN
- Molecular Weight:362.79
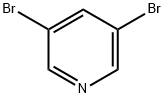
625-92-3
500 suppliers
$6.00/10g

1214383-75-1
26 suppliers
inquiry
Yield:1214383-75-1 70%
Reaction Conditions:
Stage #1: 3,5-dibromopyridinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -85; for 0.75 h;Inert atmosphere;Molecular sieve;Negishi Coupling;
Stage #2: with iodine in tetrahydrofuran;hexane at -78; for 4 h;Inert atmosphere;Molecular sieve;Negishi Coupling;
Steps:
To a solution of distilled diisopropylamine (232 μL, 1.64 mmol) in THF (2.25 mL) was dropwisely added n-butyllithium (1.6 M in hexanes; 1.0 mL, 1.64 mmol) at -78 °C. The resulting lithium diisopropylamine (LDA) solution was stirred at -78 °C for 0.5 h to allow for completion of the reaction, and subsequently cooled to -85 °C. The LDA solution was then syringed into the solution of 3,5-diboromopyridine 11 (300 mg, 1.26 mmol) in THF (0.3 mL), and stirred at -85 °C for 45 min, until becoming a deep maroon solution. To the solution of lithiated pyridine was dropwisely added iodine (642 mg, 2.53 mmol) in THF (0.6 mL) at -80 °C. By the end of the addition, the solution became dark brown. The mixture was stirred at -78 °C for a further 4 h, and then allowed to warm to room temperature overnight. The reaction mixture was then diluted with EtOAc, quenched with water, and washed with NaHSO3. The aqueous layer was then extracted with EtOAc. The combined organic layers were washed with brine, dried over Na2SO4, and then concentrated in vacuo to give the crude product as a yellow solid. The remaining solid was purified by silica gel column chromatography three times, eluting with hexane/EtOAc =2/1. The obtained white solid was recrystallized from benzene to give a colorless solid 5b.
References:
Yanuma, Hiroto;Usuki, Toyonobu [Tetrahedron Letters,2012,vol. 53,# 44,p. 5920 - 5922] Location in patent:supporting information
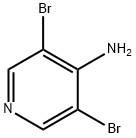
84539-34-4
174 suppliers
$6.00/1g

1214383-75-1
26 suppliers
inquiry
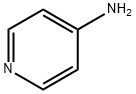
504-24-5
487 suppliers
$10.00/1g

1214383-75-1
26 suppliers
inquiry