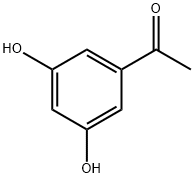
3,5-Dihydroxyacetophenone synthesis
- Product Name:3,5-Dihydroxyacetophenone
- CAS Number:51863-60-6
- Molecular formula:C8H8O3
- Molecular Weight:152.15
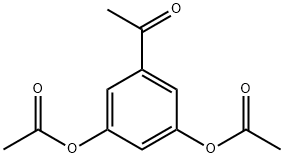
35086-59-0
209 suppliers
$5.00/1g

51863-60-6
328 suppliers
$14.00/5g
Yield:-
Reaction Conditions:
with immobilized Burkholderia cepacia lipase (Amano PS-IM) in tetrahydrofuran;isopropyl alcohol at 20; for 60 h;Inert atmosphere;Enzymatic reaction;
Steps:
1 2.1 1-(3,5-Dihydroxyphenyl)ethanone (3a)
For a preparative scale-reaction, the commercially available 3b (5.32 g, 22.5 mmol) showed smell of acetic acid, and prior to use, it was evacuated in a 200-mL brown-colored recovery flask with for 30 min at 20 mmHg. Then it was diluted with a mixture of 2-propanol (25 mL) and tetrahydrofuran (THF, 50 mL) and lipase PS-IM (2.5 g) was added. The mixture was stirred for 60 h at room temperature under argon atmosphere. After recovery of lipase by suction filtration, the filtrate was concentrated in vacuo to give 3a (3.52 g, quant) as solid. 1H NMR (500 MHz, CDCl3): δ 2.55 (s, 3H), 6.56 (t, J = 2.5 Hz, 1H), 6.99 (d, J = 2.5 Hz, 2H). Its NMR spectrum was identical with that of an authentic sample. This was employed for the next step without further purification. The recovered lipase was effectively active, after storage in a refrigerator for 2 months.
References:
Asami, Kento;Machida, Takuya;Jung, Sonna;Hanaya, Kengo;Shoji, Mitsuru;Sugai, Takeshi [Journal of Molecular Catalysis B: Enzymatic,2013,vol. 97,p. 106 - 109]
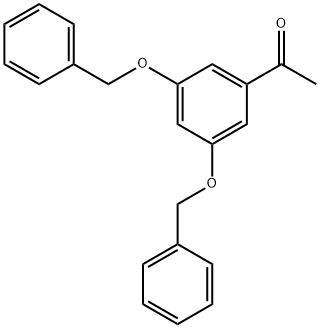
28924-21-2
345 suppliers
$22.00/5g

51863-60-6
328 suppliers
$14.00/5g
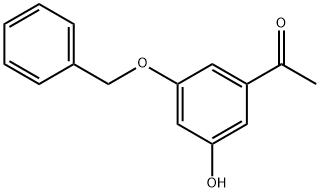
81732-54-9
18 suppliers
inquiry
50513-72-9
26 suppliers
inquiry

51863-60-6
328 suppliers
$14.00/5g
![Ethanone, 1-[3,5-bis(phenylmethoxy)phenyl]-2-(methylsulfonyl)-](/CAS/20210305/GIF/90664-17-8.gif)
90664-17-8
0 suppliers
inquiry

51863-60-6
328 suppliers
$14.00/5g
39151-19-4
202 suppliers
$17.00/250mg

51863-60-6
328 suppliers
$14.00/5g