
3-ALLYLINDENE synthesis
- Product Name:3-ALLYLINDENE
- CAS Number:2294-87-3
- Molecular formula:C12H12
- Molecular Weight:156.22
Yield:-
Reaction Conditions:
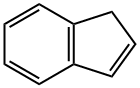
Stage #1: 1-indenewith n-butyllithium in tetrahydrofuran;hexane; for 20 h;Cooling with ice;
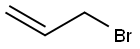
Stage #2: allyl bromide in tetrahydrofuran;hexane; for 48 h;Cooling with ice;
Steps:
4 EXAMPLES 3-6 Synthesis of substituted indenes
In Example 4, 3-(2-propenyl)indene was prepared by dissolving 15 mL (129 mmol) of indene (90%) in 50 mL of THF, and cooling in dry ice. A solution of n- butyllithium in hexanes (57 mL of 2.5 M) was added dropwise. The mixture became very thick near the end of the addition, and the ice bath was removed. The mixture was stirred for 20 hr, and then added by cannula over 30 min to 11.7 mL of allyl bromide (135 mmol) in 25 mL of THF, while cooling in ice. The solution was stirred for 48 hr and then cooled while 200 mL of 1M HC1 were added. The mixture was extracted with 100 mL of pentane, then the extract was washed with 3 x 100 mL of water, and then reduced to an oil on a rotary evaporator. The oil was dissolved in pentane and dried over sodium sulfate and filtered. The solvent was then removed under vacuum, resulting in a yellow liquid. Analysis of the yellow liquid by GC/MS showed 85.7% 3- propenylindene and 4.37% 1-propenylindene, along with minor contaminants.
References:
WO2013/19769,2013,A1 Location in patent:Page/Page column 59
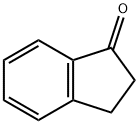
83-33-0
575 suppliers
$6.00/5g

2294-87-3
5 suppliers
inquiry
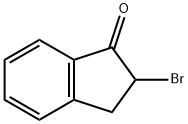
1775-27-5
112 suppliers
$14.14/250mgs:

2294-87-3
5 suppliers
inquiry
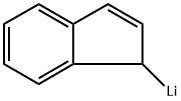
20669-47-0
0 suppliers
inquiry
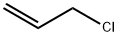
107-05-1
427 suppliers
$16.80/100ML

2294-87-3
5 suppliers
inquiry

20258-77-9
4 suppliers
inquiry
![Spiro[1,3-dithiolane-2,1'-[1H]indene], 2',3'-dihydro-](/CAS/20210305/GIF/172-16-7.gif)