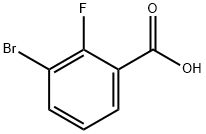
3-Bromo-2-fluorobenzoic acid synthesis
- Product Name:3-Bromo-2-fluorobenzoic acid
- CAS Number:161957-56-8
- Molecular formula:C7H4BrFO2
- Molecular Weight:219.01
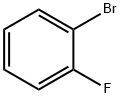
1072-85-1

124-38-9

161957-56-8
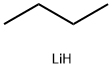
1. n-Butyl lithium (n-BuLi, 240 mL of a 2.5 M solution, 600 mmol) was added dropwise to a tetrahydrofuran (THF, 500 mL) solution of diisopropylamine (84.3 mL, 600 mmol) at -30°C. 2. The resulting solution was stirred at -30°C for 30 minutes. 3. The mixture was added dropwise to a solution of 1-bromo-2-fluorobenzene (100 g, 571.43 mmol) in THF (500 mL) at -78° C. The solution was stirred for 30 minutes at -78° C. The mixture was then added to a solution of 1-bromo-2-fluorobenzene (100 g, 571.43 mmol). 4. After the addition, the resulting solution was stirred at -78°C for 2 hours. 5. The reaction mixture was poured over crushed solid carbon dioxide. 6. After removal of the solvent in vacuo, the resulting residue was diluted with water and washed with ether (2 x 500 mL). 7. The aqueous phase was acidified to pH 2 with 1N hydrochloric acid (HCl). 8. The precipitate was collected by filtration and dried to give 3-bromo-2-fluorobenzoic acid (90 g, 64% yield) as a white solid. 9. Mass spectrometry (MS) analysis showed m/z 221.0 (M + H+).

1072-85-1
468 suppliers
$5.00/10g

124-38-9
134 suppliers
$214.00/14L

161957-56-8
280 suppliers
$6.00/1g
Yield:161957-56-8 64%
Reaction Conditions:
Stage #1: o-fluorobromobenzenewith n-butyllithium;N-ethyl-N,N-diisopropylamine in tetrahydrofuran at -78; for 2 h;
Stage #2: carbon dioxide in tetrahydrofuran;
Stage #3: with hydrogenchloride in water; pH=2;
Steps:
7
INTERMEDIATE PREPARATION 76-bromo-7-fluoro-1 /-/-indole-2,3-dione3-bromo-2-fluorobenzoic acidTo a solution of diisopropylamine (84.3 mL, 600 mmol) in THF (500 mL) at -30°C was added n-BuLi (240 mL of a 2.5M solution, 600 mmol) dropwise. The resulting solution was stirred at -30°C for 30 min. This mixture was added dropwise to a solution of 1-bromo-2- fluorobenzene (100 g, 571 .43 mmol) in THF (500 mL) at -78°C. After the addition was complete, the resulting solution was stirred at -78°C for 2 h and poured over crushed solid C02. After removal of the solvent in vacuo, the resulting residue was diluted with water and washed with diethyl ether (2 x 500 mL). The aqueous phase was acidified with 1 N HCI to pH 2. The precipitate was collected by filtration and dried to afford 3-bromo-2-fluorobenzoic acid (90 g, 64% yield) as a white solid. MS (m/z) 221.0 (M+H+).
References:
WO2011/119704,2011,A1 Location in patent:Page/Page column 39-40

124-38-9
134 suppliers
$214.00/14L

161957-56-8
280 suppliers
$6.00/1g
206551-41-9
151 suppliers
$9.00/250mg

161957-56-8
280 suppliers
$6.00/1g
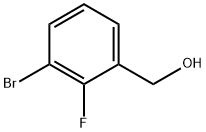
261723-32-4
140 suppliers
$10.00/1g

161957-56-8
280 suppliers
$6.00/1g