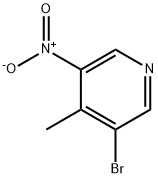
3-Bromo-4-methyl-5-nitropyridine synthesis
- Product Name:3-Bromo-4-methyl-5-nitropyridine
- CAS Number:69872-15-7
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02
31872-63-6

105-53-3

69872-15-7
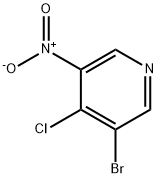
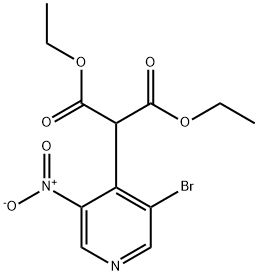
GENERAL STEPS: Diethyl malonate (3.84 mL, 25.3 mmol) was slowly added dropwise to a suspension of sodium hydride (1.01 g, 60% oil suspension, 25.3 mmol) in DMF (15 mL) at 0 °C and stirred for 30 min until gas escape ceased. Subsequently, 3-bromo-4-chloro-5-nitropyridine (3.00 g, 12.6 mmol) was slowly added and the resulting dark reddish brown solution was stirred for 1 hour at room temperature. Upon completion of the reaction, it was carefully quenched with water and the pH was adjusted to 1 with 1 N HCl. The aqueous phase was extracted with EtOAc (2 x 150 mL), the organic phases were combined and washed with deionized water (100 mL) and saturated saline, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The residue was dissolved in 4N HCl (50 mL) and refluxed for 16 hours. The reaction mixture was cooled in an ice bath and neutralized with 50% NaOH solution to pH 7. The aqueous phase was again extracted with EtOAc (3 x 100 mL), the organic phases were combined and washed with saturated saline, dried over MgSO4, filtered and concentrated under reduced pressure to afford the target product 3-bromo-4-methyl-5-nitropyridine (94a, 1.90 g, 70% yield) as a yellow solid. The product was characterized by 1H NMR (300 MHz, CDCl3): δ 8.94 (s, 1H), 8.97 (s, 1H), 2.65 (s, 3H).
Yield:69872-15-7 70%
Reaction Conditions:
Stage #1:diethyl malonate with sodium hydride in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2:3-bromo-4-chloro-5-nitropyridine in DMF (N,N-dimethyl-formamide) at 20; for 1 h;
Steps:
94 3-bromo-4-methyl-5-nitropyridine (94a)
3-bromo-4-methyl-5-nitropyridine (94a) Diethyl malonate (3.84 mL, 25.3 mmol) was slowly added to a suspension of sodium hydride (1.01 g of a 60% suspension in oil, 25.3 mmol) in DMF (15 mL) at 0° C. and stirred 30 min until gas evolution ceased. 3-bromo-4-chloro-5-nitropyridine (3.00 g, 12.6 mmol) was added slowly, and the dark reddish-brown solution was stirred at room temperature for 1 hour. The reaction was carefully quenched with water and acidified to pH 1 with 1N HCl. The aqueous mixture was extracted with EtOAc (2*150 mL). The combined organics were washed with water (100 mL) and brine, dried (Na2SO4), filtered, and concentrated in vacuo. The residue was diluted with 4N HCl (50 mL) and the solution refluxed 16 hours. The mixture was cooled in an ice bath and basified to pH 7 with 50% NaOH. The aqueous mixture was extracted with EtOAc (3*100 mL) and the combined organics were washed with brine, dried (MgSO4), filtered and concentrated in vacuo to afford 94a (1.90 g, 70%) as a yellow solid. 1H NMR (300 MHz, CDCl3) δ 8.94 (s,1H), 8.97 (s,1H), 2.65 (s, 3H).
References:
PFIZER INC US2005/90529, 2005, A1 Location in patent:Page/Page column 82
69872-14-6
6 suppliers
inquiry

69872-15-7
122 suppliers
inquiry

31872-63-6
179 suppliers
inquiry

69872-15-7
122 suppliers
inquiry
31872-65-8
141 suppliers
$12.00/250mg

69872-15-7
122 suppliers
inquiry
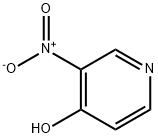
5435-54-1
364 suppliers
$19.00/25g

69872-15-7
122 suppliers
inquiry