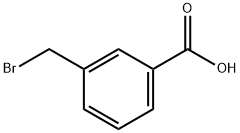
3-(BROMOMETHYL)BENZOIC ACID synthesis
- Product Name:3-(BROMOMETHYL)BENZOIC ACID
- CAS Number:6515-58-8
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
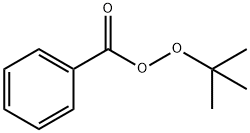
614-45-9
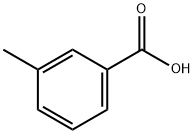
99-04-7

6515-58-8
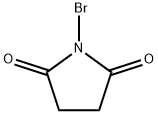
a) A mixture of m-toluic acid (15.0 g, 110 mmol), N-bromosuccinimide (19.60 g, 110 mmol) and tert-butyl peroxybenzoate (2.1 mL, 110 mmol) was mixed in carbon tetrachloride (50 mL) and heated to reflux the reaction overnight. Upon completion of the reaction, the mixture was cooled to room temperature and concentrated under reduced pressure. The resulting residue was washed with carbon tetrachloride and subsequently vacuum filtered. The filtrate was evaporated to dryness to afford the white solid product 3-bromomethylbenzoic acid (12.57 g, 53% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.93 (m, 2H), 7.43 (m, 2H), 4.55 (s, 2H).

99-04-7
480 suppliers
$8.00/100g

6515-58-8
161 suppliers
$5.00/250mg
Yield:6515-58-8 86%
Reaction Conditions:
with N-Bromosuccinimide;dibenzoyl peroxide in dichloromethane for 2 h;Reflux;
Steps:
4.1 Step 1. Preparation of 3-bromomethylbenzoic acid (Compound 10)
Methylmethylbenzoic acid (2.0 g, 14.7 mmol)And benzoyl peroxide (BPO, 0.036 g, 0.15 mmol)Was dissolved in dichloromethane (6 mL) and heated to reflux,NBS (2.6 g, 14.7 mmol) was added in portions,Continue to heat reflux 2h,After cooling to room temperature, the reaction was quenched with water (10 mL), extracted with dichloromethane (50 mL), washed with water, washed with saturated NaCl, dried over anhydrous Na2SO4,Concentrated to give a pale yellow solid 2.7 g, yield 86%
References:
Zhejiang University;Sheng Rong;Hu Yongzhou;Cao Ji;Li Shan;Qiu Ni;Zhao Mengdan;Yang Bo;He Qiaojun CN106366078, 2017, A Location in patent:Paragraph 0229; 0230; 0231

614-45-9
254 suppliers
$19.30/5g

99-04-7
480 suppliers
$8.00/100g

6515-58-8
161 suppliers
$5.00/250mg
128-08-5
894 suppliers
$5.00/5 g

99-04-7
480 suppliers
$8.00/100g
123-56-8
529 suppliers
$6.00/25g

6515-58-8
161 suppliers
$5.00/250mg

128-08-5
894 suppliers
$5.00/5 g

99-04-7
480 suppliers
$8.00/100g

6515-58-8
161 suppliers
$5.00/250mg

209743-33-9
4 suppliers
$157.00/10g

6515-58-8
161 suppliers
$5.00/250mg