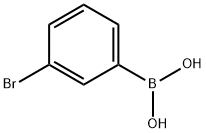
3-Bromophenylboronic acid synthesis
- Product Name:3-Bromophenylboronic acid
- CAS Number:89598-96-9
- Molecular formula:C6H6BBrO2
- Molecular Weight:200.83
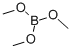
121-43-7
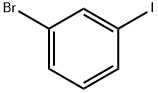
591-18-4

89598-96-9
In a 500 mL round bottom flask, 1-bromo-3-iodobenzene (25.0 g, 88 mmol) was dissolved in 200 mL of tetrahydrofuran. The reaction solution was cooled to -78°C under nitrogen protection. n-Butyllithium (60.75 mL, 97 mmol) was slowly added dropwise to the cooled solution over a period of 30 minutes and stirring was continued at the same temperature for 1 hour. Subsequently, trimethyl borate (11 g, 106 mmol) was added dropwise at the same temperature, and then the mixture was gradually warmed to room temperature and stirred overnight. Upon completion of the reaction, 2 equivalents of hydrochloric acid was added dropwise to the reaction solution for acidification and stirring was continued for 1 hour. The reaction mixture was extracted with ethyl acetate, the organic layers were combined and concentrated under reduced pressure. The resulting crystals were dissolved in cold hexane to give Intermediate 11-a (12 g, 67.6% yield).
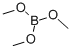
121-43-7
381 suppliers
$13.00/25mL
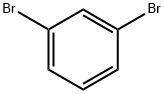
108-36-1
455 suppliers
$10.00/1g

89598-96-9
345 suppliers
$9.00/1g
Yield:89598-96-9 71.9%
Reaction Conditions:
Stage #1:1,3-dibromobenzene with n-butyllithium in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
Stage #2:Trimethyl borate in tetrahydrofuran at 20; for 3 h;
Steps:
1 Synthesis of Intermediate A-1
(105.9 mmol) of 1,3-dibromobenzene in 500 ml of nitrogen at -78 ° C under N2 atmosphereTHF for 10 minutes,then,To this was slowly added dropwise 44 ml of 2.5 M n-BuLi through a dropping funnel,The resulting solution was stirred for 30 minutes.After that,10.4 g (110 mmol) of trimethyl borate was slowly added dropwise thereto through a dropping funnel,then,The resulting solution was stirred at room temperature for 3 hours;Then, 300 ml of 1M hydrochloric acid solution was added thereto, and the extraction process was performed once. Then by usingThe organic layer separated from it was subjected to three extraction procedures with water and diethyl ether. And dried by using magnesium sulfateTo the organic layer, the residue obtained by evaporation of the solvent was isolated by silica gel column chromatography, To obtain 15.3 g (76.2 mmol, yield 71.9%) of intermediate A-1.
References:
Samsung Display Co., Ltd.;Kim, Kwanghyun;Kim, Jongwoo;Kim, Youngkook;Jun, Mieun;Hwang, Seokhwan CN105541778, 2016, A Location in patent:Paragraph 0376; 0377; 0378; 0379
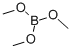
121-43-7
381 suppliers
$13.00/25mL

591-18-4
397 suppliers
$5.00/5g

89598-96-9
345 suppliers
$9.00/1g
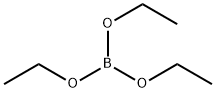
150-46-9
200 suppliers
$12.00/25mL

108-36-1
455 suppliers
$10.00/1g

89598-96-9
345 suppliers
$9.00/1g
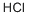
7647-01-0
0 suppliers
$10.00/10g
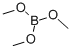
121-43-7
381 suppliers
$13.00/25mL

108-36-1
455 suppliers
$10.00/1g

89598-96-9
345 suppliers
$9.00/1g
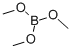
121-43-7
381 suppliers
$13.00/25mL

591-18-4
397 suppliers
$5.00/5g
7732-18-5
507 suppliers
$12.69/100ml

89598-96-9
345 suppliers
$9.00/1g