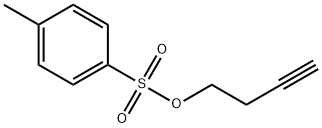
3-Butynyl p-toluenesulfonate synthesis
- Product Name:3-Butynyl p-toluenesulfonate
- CAS Number:23418-85-1
- Molecular formula:C11H12O3S
- Molecular Weight:224.28
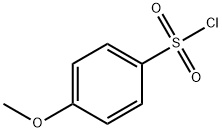
98-59-9

927-74-2

23418-85-1
A hexane solution of n-butyllithium (n-BuLi, 2.80 mL, 2.60 M, 7.27 mmol) was slowly added dropwise to a tetrahydrofuran (THF, 7.5 mL) solution of 3-butyn-1-ol (0.50 mL, 6.61 mmol) at -78 °C. The reaction mixture was kept at -78 °C for 5 min before warming up to 0 °C and continued stirring for 30 min. Subsequently, a solution of p-Toluenesulfonyl chloride (p-TsCl, 1.51 g, 7.93 mmol) in THF (8 mL) was added and the reaction continued to be stirred at 0 °C for 30 min. Upon completion of the reaction, the reaction was quenched by the addition of water (2 mL) and the mixture was extracted with ether (Et2O, 3 × 10 mL). The organic phases were combined, dried with anhydrous sodium sulfate (Na2SO4), and concentrated under reduced pressure to remove the solvent to afford 3-butynyl p-toluenesulfonate (1.41 g, 95% yield) as a light yellow oil. The product was confirmed by nuclear magnetic resonance hydrogen spectroscopy (1H NMR, 400 MHz, CDCl3): δ 7.78 (d, J = 8.0 Hz, 2H), 7.33 (d, J = 8.0 Hz, 2H), 4.08 (t, J = 7.1 Hz, 2H), 2.53 (dt, J = 7.1, 2.6 Hz, 2H), 2.43 (s, 3H), 1.95 ( t, J = 2.6 Hz, 1H); nuclear magnetic resonance carbon spectrum (13C NMR, 100 MHz, CDCl3): δ 145.0, 132.7, 129.9, 127.9, 78.3, 70.7, 67.4, 21.6, 19.4; infrared spectra (IR, pure): 3290, 2962, 2919, 1598, 1359, 1190, 1176, 980, 904, 815 cm-1; mass spectra (ESI): m/z 247.0399 [C11H12O3S (M+23) theoretical value 247.0400], 471 (base), 247, 225.
121-44-8
904 suppliers
$9.00/5g

98-59-9
625 suppliers
$9.00/5g

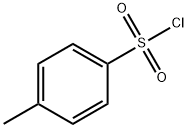
927-74-2
426 suppliers
$6.00/5g

23418-85-1
163 suppliers
$32.60/1g
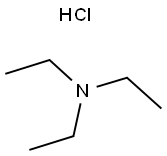
554-68-7
502 suppliers
$6.00/25g
Yield:23418-85-1 97%
Reaction Conditions:
in dichloromethane at 0 - 20; for 21 h;
Steps:
2
3-Butyn-1-ol (1.8 g, 25 mmol) was dissolved in methylene chloride (CH2CL2) (40 mL) and triethylamine (ET3N) (4.18 mL, 30 mmol). The solution was stirred at 0°C followed by addition of p-toluenesulfonyl chloride (5.05 g, 26.25 mmol). The reaction was allowed to warm to room temperature over a period of 1 hour and stirring was continued overnight. Thin layer chromatography (TLC) analysis (hexanes/ethyl acetate (EtOAc) 6: 1) after 20 hours of reaction showed a complete consumption OF 3-BUTYN-1-OL. The precipitated triethylamine HYDROCHLORIDC was filtered off and the filtrate washed with water (H20) (30 mL) and brine (30 mL). The organic layer was dried over sodium sulfate (NA2S04) and the solvent evaporated away to give 155 as a light-yellow oil (5.45 g, 97%). The crude oil was used without further purification; however, it could be purified on a silica gel column, first eluting with 8% EtOAc in hexanes followed by 40% EtOAc in hexanes.
References:
RIB-X PHARMACEUTICALS, INC. WO2004/29066, 2004, A2 Location in patent:Page/Page column 215

121-44-8
904 suppliers
$9.00/5g

98-59-9
625 suppliers
$9.00/5g

927-74-2
426 suppliers
$6.00/5g

23418-85-1
163 suppliers
$32.60/1g