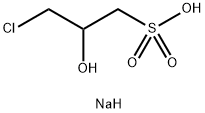
Sodium 3-Chloro-2-hydroxypropanesulfonate synthesis
- Product Name:Sodium 3-Chloro-2-hydroxypropanesulfonate
- CAS Number:126-83-0
- Molecular formula:C3H8ClNaO4S
- Molecular Weight:198.59

106-89-8
779 suppliers
$9.00/10 g

126-83-0
308 suppliers
$5.00/5g
Yield:-
Reaction Conditions:
with sodium hydrogensulfite in water
Steps:
1.A A.
A. Synthesis of 3-chloro-2-hydroxypropylsulfonic acid, sodium salt (CHPS) Epichlorohydrin (one mole, 93 g) is slowly added to an aqueous solution of one mole sodium bisulfite (104 g in about 120 ml water) while maintaining the temperature at about 80° C. The epichlorohydrin is added dropwise over about three hours, and after addition is complete, the mixture is stirred for an additional hour. After the mixture cools to about 20° C., the solids of 3-chloro-2-hydroxypropylsulfonic acid sodium salt formed from the reaction are filtered and analyzed by Nuclear Magnetic Resonance (NMR). The ISC spectra shows peaks at 69.99 ppm, 56.97 ppm, and 51.18 ppm from TMS (tetramethyl silane) and the proton spectrum shows, three groups of peaks centering at 2.95, 3.58 and 4.17 ppm (from TMS), in a 2:2:1 ratio respectively, consistent with the structure of CHPS.
References:
The Dow Chemical Company US5208369, 1993, A
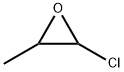
66794-22-7
0 suppliers
inquiry

126-83-0
308 suppliers
$5.00/5g