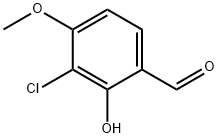
3-Chloro-4-Methoxysalicylaldehyde synthesis
- Product Name:3-Chloro-4-Methoxysalicylaldehyde
- CAS Number:72482-15-6
- Molecular formula:C8H7ClO3
- Molecular Weight:186.59

50-00-0
896 suppliers
$10.00/25g
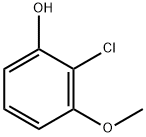
72232-49-6
99 suppliers
$22.00/250mg

72482-15-6
24 suppliers
$200.00/100mg
Yield:72482-15-6 78.6%
Reaction Conditions:
Stage #1: 2-chloro-3-methoxyphenolwith triethylamine;magnesium chloride in acetonitrile at 0; for 0.166667 h;
Stage #2: formaldehyd in acetonitrile at 0 - 75; for 4 h;
Steps:
1.1 Step 1:
A stirred solution of 2-chloro-3-methoxyphenol (10 g, 63.29 mmol, 1 eq) in ACN (200 ml_) was cooled to 0°C and MgCh (9 g, 94.8 mmol, 1 .5 eq) was added followed by TEA (32.7 mL, 233.8 mmol, 3.7 eq) and the resulting reaction mixture was stirred for 10 min. Paraformaldehyde (12.8 g, 426.6 mmol, 6.75 eq) was then added portionwise at 0°C and the mixture heated at 75 °C for 4 h. TLC analysis indicated formation of a polar spot. The reaction mixture was concentrated under reduced pressure, acidified with aqueous 2N HCI solution and extracted with ethyl acetate (2x500 mL). The combined organic layer was dried over Na2S04 and concentrated under reduced pressure. The crude product was triturated with diethyl ether to afford 3-chloro-2-hydroxy-4- methoxybenzaldehyde (9.2 g, 78.6% yield) as a pale yellow solid. LCMS: m/z 187.34 (M+H).
References:
WO2019/119145,2019,A1 Location in patent:Paragraph 00257

72482-14-5
30 suppliers
$57.00/100mg

72482-15-6
24 suppliers
$200.00/100mg
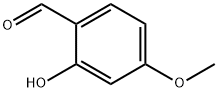
673-22-3
497 suppliers
$5.00/1g
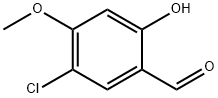
89938-56-7
25 suppliers
$225.00/1g

72482-15-6
24 suppliers
$200.00/100mg
7051-15-2
52 suppliers
$100.00/1g

72482-15-6
24 suppliers
$200.00/100mg