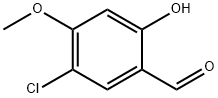
5-Chloro-4-Methoxysalicylaldehyde synthesis
- Product Name:5-Chloro-4-Methoxysalicylaldehyde
- CAS Number:89938-56-7
- Molecular formula:C8H7ClO3
- Molecular Weight:186.59
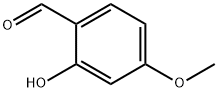
673-22-3
496 suppliers
$5.00/1g

89938-56-7
25 suppliers
$225.00/1g
Yield:89938-56-7 97.8482%
Reaction Conditions:
Stage #1: 2-Hydroxy-4-methoxybenzaldehydewith toluene-4-sulfonic acid in dichloromethane at 20; for 0.5 h;
Stage #2: with N-chloro-succinimide at 0 - 20; for 5 h;
Steps:
P1 Example P1 : Preparation of 5-chloro-2-hvdroxy-4-methoxy-benzaldehyde
Example P1 : Preparation of 5-chloro-2-hvdroxy-4-methoxy-benzaldehyde: (0345) To a stirred solution of 2-hydroxy-4-methoxy-benzaldehyde (10 g, 65.725 mmol) in dichloromethane (50 ml) was added p-toluene sulfonic acid (5.65g, 32.8625 mmol, ) at room temperature. Resulting mixture was stirred at ambient temperature for 30 minutes. Reaction mixture was cooled to 0°C and 1 -chloropyrrolidine- 2,5-dione (9.21g., 69.01 13 mmol) was added and then allowed to stir at ambient temperature for 5h. Reaction mixture was diluted with dichloromethane (50 ml). Combined organic layer was washed with sodium bicarbonate solution (4 X 20ml), and then with water (2 x 50 mL) followed by brine (50 mL). Organic layer was dried over Na2S04, filtered and evaporated completely to afford the product 5-chloro-2-hydroxy-4- methoxy-benzaldehyde (12 g, 97.8482% of theoretical yield) as a gummy liquid. H NMR (400 MHz, DMSO-d6) δ ppm 3.91 (s, 3 H) 6.68 (s, 1 H) 7.67 (s, 1 H) 10.03 (s, 1 H) 1 1.15 (s, 1 H)
References:
WO2015/121442,2015,A1 Location in patent:Page/Page column 50-51

673-22-3
496 suppliers
$5.00/1g

89938-56-7
25 suppliers
$225.00/1g
72482-15-6
24 suppliers
$200.00/100mg

50-00-0
896 suppliers
$10.00/25g
18113-07-0
53 suppliers
$21.00/100mg

89938-56-7
25 suppliers
$225.00/1g
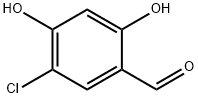
131088-02-3
62 suppliers
inquiry

74-88-4
356 suppliers
$15.00/10g

89938-56-7
25 suppliers
$225.00/1g
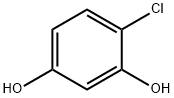
95-88-5
409 suppliers
$9.00/25g

89938-56-7
25 suppliers
$225.00/1g